Mathematical modeling of the effects of glutathione on arsenic methylation
- PMID: 24885596
- PMCID: PMC4041632
- DOI: 10.1186/1742-4682-11-20
Mathematical modeling of the effects of glutathione on arsenic methylation
Abstract
Background: Arsenic is a major environmental toxin that is detoxified in the liver by biochemical mechanisms that are still under study. In the traditional metabolic pathway, arsenic undergoes two methylation reactions, each followed by a reduction, after which it is exported and released in the urine. Recent experiments show that glutathione plays an important role in arsenic detoxification and an alternative biochemical pathway has been proposed in which arsenic is first conjugated by glutathione after which the conjugates are methylated. In addition, in rats arsenic-glutathione conjugates can be exported into the plasma and removed by the liver in the bile.
Methods: We have developed a mathematical model for arsenic biochemistry that includes three mechanisms by which glutathione affects arsenic methylation: glutathione increases the speed of the reduction steps; glutathione affects the activity of arsenic methyltranferase; glutathione sequesters inorganic arsenic and its methylated downstream products. The model is based as much as possible on the known biochemistry of arsenic methylation derived from cellular and experimental studies.
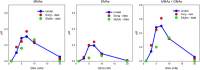
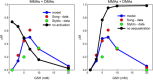
Results: We show that the model predicts and helps explain recent experimental data on the effects of glutathione on arsenic methylation. We explain why the experimental data imply that monomethyl arsonic acid inhibits the second methylation step. The model predicts time course data from recent experimental studies. We explain why increasing glutathione when it is low increases arsenic methylation and that at very high concentrations increasing glutathione decreases methylation. We explain why the possible temporal variation of the glutathione concentration affects the interpretation of experimental studies that last hours.
Conclusions: The mathematical model aids in the interpretation of data from recent experimental studies and shows that the Challenger pathway of arsenic methylation, supplemented by the glutathione effects described above, is sufficient to understand and predict recent experimental data. More experimental studies are needed to explicate the detailed mechanisms of action of glutathione on arsenic methylation. Recent experimental work on the effects of glutathione on arsenic methylation and our modeling study suggest that supplements that increase hepatic glutathione production should be considered as strategies to reduce adverse health effects in affected populations.
Figures
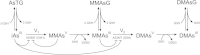



Similar articles
-
Mathematical model insights into arsenic detoxification.Theor Biol Med Model. 2011 Aug 26;8:31. doi: 10.1186/1742-4682-8-31. Theor Biol Med Model. 2011. PMID: 21871107 Free PMC article.
-
Study of factors influencing the in vivo methylation of inorganic arsenic in rats.Toxicol Appl Pharmacol. 1987 Oct;91(1):65-74. doi: 10.1016/0041-008x(87)90194-3. Toxicol Appl Pharmacol. 1987. PMID: 3672518
-
A new metabolic pathway of arsenite: arsenic-glutathione complexes are substrates for human arsenic methyltransferase Cyt19.Arch Toxicol. 2005 Apr;79(4):183-91. doi: 10.1007/s00204-004-0620-x. Epub 2004 Nov 4. Arch Toxicol. 2005. PMID: 15526190
-
Arsenic metabolism and thioarsenicals.Metallomics. 2012 Aug;4(9):881-92. doi: 10.1039/c2mt00181k. Metallomics. 2012. PMID: 22358131 Review.
-
Metabolism of arsenic and its toxicological relevance.Arch Toxicol. 2013 Jun;87(6):969-79. doi: 10.1007/s00204-012-0904-5. Epub 2012 Jul 19. Arch Toxicol. 2013. PMID: 22811022 Review.
Cited by
-
Including Rebinding Reactions in Well-Mixed Models of Distributive Biochemical Reactions.Biophys J. 2016 Nov 15;111(10):2317-2326. doi: 10.1016/j.bpj.2016.10.008. Biophys J. 2016. PMID: 27851953 Free PMC article.
-
Assessing the Impact of PM2.5-Bound Arsenic on Cardiovascular Risk among Workers in a Non-ferrous Metal Smelting Area: Insights from Chemical Speciation and Bioavailability.Environ Sci Technol. 2024 May 14;58(19):8228-8238. doi: 10.1021/acs.est.3c10761. Epub 2024 May 2. Environ Sci Technol. 2024. PMID: 38695658 Free PMC article.
-
Arsenic toxicokinetic modeling and risk analysis: Progress, needs and applications.Toxicology. 2021 Jun 15;457:152809. doi: 10.1016/j.tox.2021.152809. Epub 2021 May 7. Toxicology. 2021. PMID: 33965444 Free PMC article. Review.
-
Mathematical Models for Immunology: Current State of the Art and Future Research Directions.Bull Math Biol. 2016 Oct;78(10):2091-2134. doi: 10.1007/s11538-016-0214-9. Epub 2016 Oct 6. Bull Math Biol. 2016. PMID: 27714570 Free PMC article. Review.
-
Nutritional Influences on One-Carbon Metabolism: Effects on Arsenic Methylation and Toxicity.Annu Rev Nutr. 2018 Aug 21;38:401-429. doi: 10.1146/annurev-nutr-082117-051757. Epub 2018 May 23. Annu Rev Nutr. 2018. PMID: 29799766 Free PMC article. Review.
References
-
- Kinniburgh DG, Smedley PL. Arsenic contamination of groundwater in Bangladesh. Final report. Tech. Rep. WC/00/19, British Geological Survey, Keyworth, UK, 2001.
-
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, vanGeen A, Slavkovic V, Lolacano NJ, Cheng Z, Iftikhar H, Momotaj H, Graziono JH. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112:1329–1333. doi: 10.1289/ehp.6964. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

