From mouth to macrophage: mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis
- PMID: 24885748
- PMCID: PMC4046017
- DOI: 10.1186/1297-9716-45-54
From mouth to macrophage: mechanisms of innate immune subversion by Mycobacterium avium subsp. paratuberculosis
Abstract
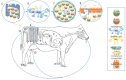
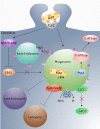
Johne's disease (JD) is a chronic enteric infection of cattle caused by Mycobacterium avium subsp. paratuberculosis (MAP). The high economic cost and potential zoonotic threat of JD have driven efforts to develop tools and approaches to effectively manage this disease within livestock herds. Efforts to control JD through traditional animal management practices are complicated by MAP's ability to cause long-term environmental contamination as well as difficulties associated with diagnosis of JD in the pre-clinical stages. As such, there is particular emphasis on the development of an effective vaccine. This is a daunting challenge, in large part due to MAP's ability to subvert protective host immune responses. Accordingly, there is a priority to understand MAP's interaction with the bovine host: this may inform rational targets and approaches for therapeutic intervention. Here we review the early host defenses encountered by MAP and the strategies employed by the pathogen to avert or subvert these responses, during the critical period between ingestion and the establishment of persistent infection in macrophages.
Figures


Similar articles
-
Transcriptome Profiling of Bovine Macrophages Infected by Mycobacterium avium spp. paratuberculosis Depicts Foam Cell and Innate Immune Tolerance Phenotypes.Front Immunol. 2020 Jan 8;10:2874. doi: 10.3389/fimmu.2019.02874. eCollection 2019. Front Immunol. 2020. PMID: 31969876 Free PMC article.
-
Mycobacterium avium subsp. paratuberculosis inhibits gamma interferon-induced signaling in bovine monocytes: insights into the cellular mechanisms of Johne's disease.Infect Immun. 2012 Sep;80(9):3039-48. doi: 10.1128/IAI.00406-12. Epub 2012 Jun 11. Infect Immun. 2012. PMID: 22689821 Free PMC article.
-
Johne's disease in cattle: an in vitro model to study early response to infection of Mycobacterium avium subsp. paratuberculosis using RNA-seq.Mol Immunol. 2017 Nov;91:259-271. doi: 10.1016/j.molimm.2017.08.017. Epub 2017 Oct 5. Mol Immunol. 2017. PMID: 28988040
-
Mycobacterium paratuberculosis and the bovine immune system.Anim Health Res Rev. 2001 Dec;2(2):141-61. Anim Health Res Rev. 2001. PMID: 11831436 Review.
-
Inflammatory Th17 responses to infection with Mycobacterium avium subspecies paratuberculosis (MAP) in cattle and their potential role in development of Johne's disease.Vet Immunol Immunopathol. 2019 Dec;218:109954. doi: 10.1016/j.vetimm.2019.109954. Epub 2019 Oct 1. Vet Immunol Immunopathol. 2019. PMID: 31733610 Review.
Cited by
-
Identification of essential genes in Mycobacterium avium subsp. paratuberculosis genome for persistence in dairy calves.Front Microbiol. 2022 Oct 20;13:994421. doi: 10.3389/fmicb.2022.994421. eCollection 2022. Front Microbiol. 2022. PMID: 36338087 Free PMC article.
-
Fasciola hepatica products can alter the response of bovine immune cells to Mycobacterium avium subsp. paratuberculosis.Parasite Immunol. 2020 Nov;42(11):e12779. doi: 10.1111/pim.12779. Epub 2020 Aug 13. Parasite Immunol. 2020. PMID: 32725900 Free PMC article.
-
Bacteria-Host Interactions in Multiple Sclerosis.Front Microbiol. 2018 Dec 4;9:2966. doi: 10.3389/fmicb.2018.02966. eCollection 2018. Front Microbiol. 2018. PMID: 30564215 Free PMC article. Review.
-
Bovine Immunity and Vitamin D3: An Emerging Association in Johne's Disease.Microorganisms. 2022 Sep 18;10(9):1865. doi: 10.3390/microorganisms10091865. Microorganisms. 2022. PMID: 36144467 Free PMC article. Review.
-
The Development of 3D Bovine Intestinal Organoid Derived Models to Investigate Mycobacterium Avium ssp Paratuberculosis Pathogenesis.Front Vet Sci. 2022 Jul 4;9:921160. doi: 10.3389/fvets.2022.921160. eCollection 2022. Front Vet Sci. 2022. PMID: 35859809 Free PMC article.
References
-
- Rohde RF, Shulaw WP. Isolation of Mycobacterium paratuberculosis from the uterine flush fluids of cows with clinical paratuberculosis. J Am Vet Med Assoc. 1990;197:1482–1483. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

