A kinetic fluorescence assay reveals unusual features of Ca⁺⁺ uptake in Plasmodium falciparum-infected erythrocytes
- PMID: 24885754
- PMCID: PMC4078004
- DOI: 10.1186/1475-2875-13-184
A kinetic fluorescence assay reveals unusual features of Ca⁺⁺ uptake in Plasmodium falciparum-infected erythrocytes
Abstract
Background: To facilitate development within erythrocytes, malaria parasites increase their host cell uptake of diverse solutes including Ca++. The mechanism and molecular basis of increased Ca++ permeability remains less well studied than that of other solutes.
Methods: Based on an appropriate Ca++ affinity and its greater brightness than related fluorophores, Fluo-8 was selected and used to develop a robust fluorescence-based assay for Ca++ uptake by human erythrocytes infected with Plasmodium falciparum.
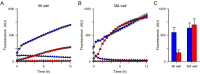
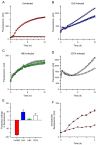
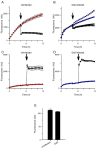
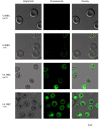
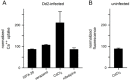
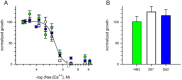
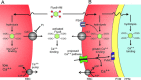
Results: Both uninfected and infected cells exhibited a large Ca++-dependent fluorescence signal after loading with the Fluo-8 dye. Probenecid, an inhibitor of erythrocyte organic anion transporters, abolished the fluorescence signal in uninfected cells; in infected cells, this agent increased fluorescence via mechanisms that depend on parasite genotype. Kinetic fluorescence measurements in 384-well microplates revealed that the infected cell Ca++ uptake is not mediated by the plasmodial surface anion channel (PSAC), a parasite nutrient channel at the host membrane; it also appears to be distinct from mammalian Ca++ channels. Imaging studies confirmed a low intracellular Ca++ in uninfected cells and higher levels in both the host and parasite compartments of infected cells. Parasite growth inhibition studies revealed a conserved requirement for extracellular Ca++.
Conclusions: Nondestructive loading of Fluo-8 into human erythrocytes permits measurement of Ca++ uptake kinetics. The greater Ca++ permeability of cells infected with malaria parasites is apparent when probenecid is used to inhibit Fluo-8 efflux at the host membrane. This permeability is mediated by a distinct pathway and may be essential for intracellular parasite development. The miniaturized assay presented here should help clarify the precise transport mechanism and may identify inhibitors suitable for antimalarial drug development.
Figures
Similar articles
-
Increased Ca++ uptake by erythrocytes infected with malaria parasites: Evidence for exported proteins and novel inhibitors.Cell Microbiol. 2018 Sep;20(9):e12853. doi: 10.1111/cmi.12853. Epub 2018 May 21. Cell Microbiol. 2018. PMID: 29726084 Free PMC article.
-
The plasmodial surface anion channel is functionally conserved in divergent malaria parasites.Eukaryot Cell. 2005 Dec;4(12):2153-9. doi: 10.1128/EC.4.12.2153-2159.2005. Eukaryot Cell. 2005. PMID: 16339732 Free PMC article.
-
Live-Cell FRET Reveals that Malaria Nutrient Channel Proteins CLAG3 and RhopH2 Remain Associated throughout Their Tortuous Trafficking.mBio. 2020 Sep 8;11(5):e01354-20. doi: 10.1128/mBio.01354-20. mBio. 2020. PMID: 32900800 Free PMC article.
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
-
Ion and nutrient uptake by malaria parasite-infected erythrocytes.Cell Microbiol. 2012 Jul;14(7):1003-9. doi: 10.1111/j.1462-5822.2012.01790.x. Epub 2012 Apr 19. Cell Microbiol. 2012. PMID: 22432505 Free PMC article. Review.
Cited by
-
Of membranes and malaria: phospholipid asymmetry in Plasmodium falciparum-infected red blood cells.Cell Mol Life Sci. 2021 May;78(10):4545-4561. doi: 10.1007/s00018-021-03799-6. Epub 2021 Mar 13. Cell Mol Life Sci. 2021. PMID: 33713154 Free PMC article. Review.
-
Complex nutrient channel phenotypes despite Mendelian inheritance in a Plasmodium falciparum genetic cross.PLoS Pathog. 2020 Feb 18;16(2):e1008363. doi: 10.1371/journal.ppat.1008363. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32069335 Free PMC article.
-
ATPe Dynamics in Protozoan Parasites. Adapt or Perish.Genes (Basel). 2018 Dec 27;10(1):16. doi: 10.3390/genes10010016. Genes (Basel). 2018. PMID: 30591699 Free PMC article. Review.
-
Unique Properties of Nutrient Channels on Plasmodium-Infected Erythrocytes.Pathogens. 2023 Oct 2;12(10):1211. doi: 10.3390/pathogens12101211. Pathogens. 2023. PMID: 37887727 Free PMC article. Review.
-
Breakdown in membrane asymmetry regulation leads to monocyte recognition of P. falciparum-infected red blood cells.PLoS Pathog. 2021 Feb 18;17(2):e1009259. doi: 10.1371/journal.ppat.1009259. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33600495 Free PMC article.
References
-
- Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–291. - PubMed
-
- Ginsburg H, Kutner S, Zangwil M, Cabantchik ZI. Selectivity properties of pores induced in host erythrocyte membrane by Plasmodium falciparum. Effect of parasite maturation. Biochim Biophys Acta. 1986;861:194–196. - PubMed
-
- Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, Ginsburg H, Hill DA, Huber SM, Kirk K, Lang F, Lisk G, Oteng E, Pillai AD, Rayavara K, Rouhani S, Saliba KJ, Shen C, Solomon T, Thomas SL, Verloo P, Desai SA. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

