Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma
- PMID: 24885819
- PMCID: PMC4113220
- DOI: 10.1186/1479-5876-12-119
Phage idiotype vaccination: first phase I/II clinical trial in patients with multiple myeloma
Abstract
Background: Multiple myeloma is characterized by clonal expansion of B cells producing monoclonal immunoglobulins or fragments thereof, which can be detected in the serum and/or urine and are ideal target antigens for patient-specific immunotherapies.
Methods: Using phage particles as immunological carriers, we employed a novel chemically linked idiotype vaccine in a clinical phase I/II trial including 15 patients with advanced multiple myeloma. Vaccines composed of purified paraproteins linked to phage were manufactured successfully for each patient. Patients received six intradermal immunizations with phage idiotype vaccines in three different dose groups.
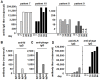
Results: Phage idiotype was well tolerated by all study participants. A subset of patients (80% in the middle dose group) displayed a clinical response indicated by decrease or stabilization of paraprotein levels. Patients exhibiting a clinical response to phage vaccines also raised idiotype-specific immunoglobulins. Induction of a cellular immune response was demonstrated by a cytotoxicity assay and delayed type hypersensitivity tests.
Conclusion: We present a simple, time- and cost-efficient phage idiotype vaccination strategy, which represents a safe and feasible patient-specific therapy for patients with advanced multiple myeloma and produced promising anti-tumor activity in a subset of patients.
Figures

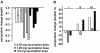
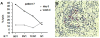
Similar articles
-
Chemically linked phage idiotype vaccination in the murine B cell lymphoma 1 model.J Transl Med. 2013 Oct 23;11:267. doi: 10.1186/1479-5876-11-267. J Transl Med. 2013. PMID: 24152874 Free PMC article.
-
Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects.Br J Haematol. 2000 Mar;108(4):805-16. doi: 10.1046/j.1365-2141.2000.01958.x. Br J Haematol. 2000. PMID: 10792287
-
Specific detection of myeloma plasma cells using anti-idiotypic single chain antibody fragments selected from a phage display library.Leukemia. 1998 Aug;12(8):1295-302. doi: 10.1038/sj.leu.2401123. Leukemia. 1998. PMID: 9697887
-
The role of idiotype vaccines in the treatment of human B-cell malignancies.Expert Rev Vaccines. 2004 Apr;3(2):163-70. doi: 10.1586/14760584.3.2.163. Expert Rev Vaccines. 2004. PMID: 15056042 Review.
-
Vaccine therapy for B-cell lymphomas: next-generation strategies.Hematology Am Soc Hematol Educ Program. 2007:243-9. doi: 10.1182/asheducation-2007.1.243. Hematology Am Soc Hematol Educ Program. 2007. PMID: 18024636 Review.
Cited by
-
Epitopes and Mimotopes Identification Using Phage Display for Vaccine Development against Infectious Pathogens.Vaccines (Basel). 2023 Jun 29;11(7):1176. doi: 10.3390/vaccines11071176. Vaccines (Basel). 2023. PMID: 37514992 Free PMC article. Review.
-
Beyond phage display: non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold.Front Microbiol. 2015 Aug 4;6:755. doi: 10.3389/fmicb.2015.00755. eCollection 2015. Front Microbiol. 2015. PMID: 26300850 Free PMC article. Review.
-
Immunotherapy for Multiple Myeloma, Past, Present, and Future: Monoclonal Antibodies, Vaccines, and Cellular Therapies.Curr Hematol Malig Rep. 2015 Dec;10(4):395-404. doi: 10.1007/s11899-015-0283-0. Curr Hematol Malig Rep. 2015. PMID: 26338470 Review.
-
Arming Filamentous Bacteriophage, a Nature-Made Nanoparticle, for New Vaccine and Immunotherapeutic Strategies.Pharmaceutics. 2019 Sep 1;11(9):437. doi: 10.3390/pharmaceutics11090437. Pharmaceutics. 2019. PMID: 31480551 Free PMC article. Review.
-
Microbial Delivery Vehicles for Allergens and Allergen-Derived Peptides in Immunotherapy of Allergic Diseases.Front Microbiol. 2018 Jul 2;9:1449. doi: 10.3389/fmicb.2018.01449. eCollection 2018. Front Microbiol. 2018. PMID: 30013543 Free PMC article. Review.
References
-
- Bendandi M, Gocke CD, Kobrin CB, Benko FA, Sternas LA, Pennington R, Watson TM, Reynolds CW, Gause BL, Duffey PL, Jaffe ES, Creekmore SP, Longo DL, Kwak LW. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

