Variable versus conventional lung protective mechanical ventilation during open abdominal surgery: study protocol for a randomized controlled trial
- PMID: 24885921
- PMCID: PMC4026052
- DOI: 10.1186/1745-6215-15-155
Variable versus conventional lung protective mechanical ventilation during open abdominal surgery: study protocol for a randomized controlled trial
Abstract
Background: General anesthesia usually requires mechanical ventilation, which is traditionally accomplished with constant tidal volumes in volume- or pressure-controlled modes. Experimental studies suggest that the use of variable tidal volumes (variable ventilation) recruits lung tissue, improves pulmonary function and reduces systemic inflammatory response. However, it is currently not known whether patients undergoing open abdominal surgery might benefit from intraoperative variable ventilation.
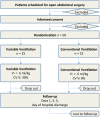
Methods/design: The PROtective VARiable ventilation trial ('PROVAR') is a single center, randomized controlled trial enrolling 50 patients who are planning for open abdominal surgery expected to last longer than 3 hours. PROVAR compares conventional (non-variable) lung protective ventilation (CV) with variable lung protective ventilation (VV) regarding pulmonary function and inflammatory response. The primary endpoint of the study is the forced vital capacity on the first postoperative day. Secondary endpoints include further lung function tests, plasma cytokine levels, spatial distribution of ventilation assessed by means of electrical impedance tomography and postoperative pulmonary complications.
Discussion: We hypothesize that VV improves lung function and reduces systemic inflammatory response compared to CV in patients receiving mechanical ventilation during general anesthesia for open abdominal surgery longer than 3 hours. PROVAR is the first randomized controlled trial aiming at intra- and postoperative effects of VV on lung function. This study may help to define the role of VV during general anesthesia requiring mechanical ventilation.
Trial registration: Clinicaltrials.gov NCT01683578 (registered on September 3 3012).
Figures
References
-
- Xue FS, Li BW, Zhang GS, Liao X, Zhang YM, Liu JH, An G, Luo LK. The influence of surgical sites on early postoperative hypoxemia in adults undergoing elective surgery. Anesth Analg. 1999;88:213–219. - PubMed
-
- Golfieri R, Giampalma E, Morselli Labate AM, D’ Arienzo P, Jovine E, Grazi GL, Mazziotti A, Maffei M, Muzzi C, Tancioni S, Sama C, Cavallari A, Gavelli G. Pulmonary complications of liver transplantation: radiological appearance and statistical evaluation of risk factors in 300 cases. Eur Radiol. 2000;10:1169–1183. doi: 10.1007/s003309900268. - DOI - PMC - PubMed
-
- van Kaam AH, Lachmann RA, Herting E, De Jaegere A, van Iwaarden F, Noorduyn LA, Kok JH, Haitsma JJ, Lachmann B. Reducing atelectasis attenuates bacterial growth and translocation in experimental pneumonia. Am J Respir Crit Care Med. 2004;169:1046–1053. doi: 10.1164/rccm.200312-1779OC. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical