Multikinase inhibitor sorafenib prevents pressure overload-induced left ventricular hypertrophy in rats by blocking the c-Raf/ERK1/2 signaling pathway
- PMID: 24885948
- PMCID: PMC4042218
- DOI: 10.1186/1749-8090-9-81
Multikinase inhibitor sorafenib prevents pressure overload-induced left ventricular hypertrophy in rats by blocking the c-Raf/ERK1/2 signaling pathway
Abstract
Background: Left ventricular hypertrophy (LVH) is a potent risk factor for sudden death and congestive heart failure.
Methods: We tested the effect of sorafenib, a multikinase inhibitor (10 mg/kg, given orally, starting 2 days prior to banding, till sacrifice on day 14), on the development of LVH following aortic banding in rats.
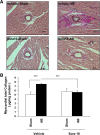
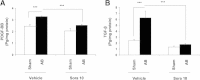
Results: The latter resulted in significant LVH caused by both an increase in cardiomyocyte volume and interstitial collagen deposition. The observed LVH was entirely blocked by sorafenib downregulating both of these components. LVH was associated with PDGF-BB and TGFβ1 overexpression, as well as phosphorylation of c-raf and ERK1/2. Additionally, the transcription factors c-myc and c-fos leading to proliferation as well as the hypertrophy-inducing transcription factor GATA4 and its regulated gene ANP were all upregulated in response to aortic banding. All these overexpressions and upregulations were inhibited upon sorafenib treatment.
Conclusion: We show that sorafenib exhibits a regulatory role on the occurrence of LVH following AB in rats by blocking the rise in growth factors PDGF-BB and TGFβ1, activation of the corresponding c-Raf-ERK1/2 signaling pathway and effector mechanisms, including GATA4 and ANP. This effect of sorafenib may be of clinical importance in modulating the maladaptive hypertrophic response to pressure overload.
Figures





Similar articles
-
Distinct antifibrogenic effects of erlotinib, sunitinib and sorafenib on rat pancreatic stellate cells.World J Gastroenterol. 2014 Jun 28;20(24):7914-25. doi: 10.3748/wjg.v20.i24.7914. World J Gastroenterol. 2014. PMID: 24976727 Free PMC article.
-
Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling.Circulation. 2008 Nov 11;118(20):2081-90. doi: 10.1161/CIRCULATIONAHA.108.779751. Epub 2008 Oct 27. Circulation. 2008. PMID: 18955668
-
Survival pathway of cholangiocarcinoma via AKT/mTOR signaling to escape RAF/MEK/ERK pathway inhibition by sorafenib.Oncol Rep. 2018 Feb;39(2):843-850. doi: 10.3892/or.2017.6153. Epub 2017 Dec 13. Oncol Rep. 2018. PMID: 29251327
-
Targeted treatment of ovarian cancer--the multiple - kinase - inhibitor sorafenib as a potential option.Anticancer Res. 2014 Apr;34(4):1519-30. Anticancer Res. 2014. PMID: 24692678 Review.
-
Kruppel-Like Factor 15 Is Critical for the Development of Left Ventricular Hypertrophy.Int J Mol Sci. 2018 Apr 27;19(5):1303. doi: 10.3390/ijms19051303. Int J Mol Sci. 2018. PMID: 29702551 Free PMC article. Review.
Cited by
-
The Traditional Chinese Medicine Gedan Jiangya Decoction Alleviates Left Ventricular Hypertrophy via Suppressing the Ras/ERK1/2 Signaling Pathway.Evid Based Complement Alternat Med. 2022 Nov 18;2022:6924197. doi: 10.1155/2022/6924197. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36437833 Free PMC article.
-
Kinetic mRNA Profiling in a Rat Model of Left-Ventricular Hypertrophy Reveals Early Expression of Chemokines and Their Receptors.PLoS One. 2016 Aug 15;11(8):e0161273. doi: 10.1371/journal.pone.0161273. eCollection 2016. PLoS One. 2016. PMID: 27525724 Free PMC article.
-
Virtual drug screen reveals context-dependent inhibition of cardiomyocyte hypertrophy.Br J Pharmacol. 2023 Nov;180(21):2721-2735. doi: 10.1111/bph.16163. Epub 2023 Jul 5. Br J Pharmacol. 2023. PMID: 37302817 Free PMC article.
-
Inhibition of the NFATc4/ERK/AKT Pathway and Improvement of Thiol-Specific Oxidative Stress by Dronedarone Possibly Secondary to the Reduction of Blood Pressure in an Animal Model of Ventricular Hypertrophy.Front Physiol. 2020 Aug 26;11:967. doi: 10.3389/fphys.2020.00967. eCollection 2020. Front Physiol. 2020. PMID: 32982770 Free PMC article.
-
Protective effect of hydrogen-rich saline on pressure overload-induced cardiac hypertrophyin rats: possible role of JAK-STAT signaling.BMC Cardiovasc Disord. 2018 Feb 13;18(1):32. doi: 10.1186/s12872-018-0773-9. BMC Cardiovasc Disord. 2018. PMID: 29433438 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

