Identification of boron-deficiency-responsive microRNAs in Citrus sinensis roots by Illumina sequencing
- PMID: 24885979
- PMCID: PMC4041134
- DOI: 10.1186/1471-2229-14-123
Identification of boron-deficiency-responsive microRNAs in Citrus sinensis roots by Illumina sequencing
Abstract
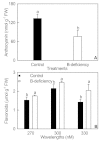
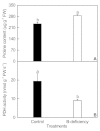
Background: Boron (B)-deficiency is a widespread problem in many crops, including Citrus. MicroRNAs (miRNAs) play important roles in nutrient deficiencies. However, little is known on B-deficiency-responsive miRNAs in plants. In this study, we first identified miRNAs and their expression pattern in B-deficient Citrus sinensis roots by Illumina sequencing in order to identify miRNAs that might be involved in the tolerance of plants to B-deficiency.
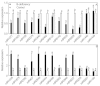
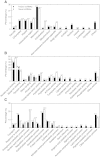
Results: We isolated 52 (40 known and 12 novel) up-regulated and 82 (72 known and 10 novel) down-regulated miRNAs from B-deficient roots, demonstrating remarkable metabolic flexibility of roots, which might contribute to the tolerance of plants to B-deficiency. A model for the possible roles of miRNAs in the tolerance of roots to B-deficiency was proposed. miRNAs might regulate the adaptations of roots to B-deficiency through following several aspects: (a) inactivating reactive oxygen species (ROS) signaling and scavenging through up-regulating miR474 and down-regulating miR782 and miR843; (b) increasing lateral root number by lowering miR5023 expression and maintaining a certain phenotype favorable for B-deficiency-tolerance by increasing miR394 expression; (c) enhancing cell transport by decreasing the transcripts of miR830, miR5266 and miR3465; (d) improving osmoprotection (miR474) and regulating other metabolic reactions (miR5023 and miR821). Other miRNAs such as miR472 and miR2118 in roots increased in response to B-deficiency, thus decreasing the expression of their target genes, which are involved in disease resistance, and hence, the disease resistance of roots.
Conclusions: Our work demonstrates the possible roles of miRNAs and related mechanisms in the response of plant roots to B-deficiency.
Figures
References
-
- Shorrocks VM. The occurrence and correction of boron deficiency. Plant Soil. 1997;193:121–148. doi: 10.1023/A:1004216126069. - DOI
-
- Xu FS, Zeng CY, Peng LS, Shi L. Analysis of gene expression profile in response to low boron stress in Arabidopsis thaliana. Acta Agri Univ Jiangxiensis. 2010;32:1004–1009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

