Systematic analysis of the factors that adversely affect the rate of cell accumulation in mouse embryos during their culture in vitro
- PMID: 24885989
- PMCID: PMC4036297
- DOI: 10.1186/1477-7827-12-35
Systematic analysis of the factors that adversely affect the rate of cell accumulation in mouse embryos during their culture in vitro
Abstract
Background: Retarded embryo growth is a pervasive effect of culture in vitro.
Methods: A systematic analysis of the interactions between media design, embryo culture density, oxygen tension, amino acids, trophic ligands and the genetic background of the mouse on embryo growth rates in vitro was performed.
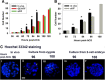
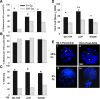
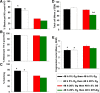
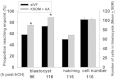
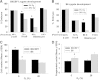
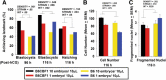
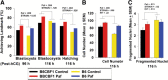
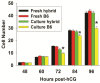
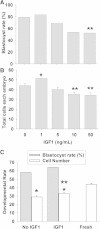
Results: Growth retardation of mouse zygotes was greater in 20% O2 than 5%, a sequential media design was superior to static simple media designs, but the supplementation of simple media with mixed amino acids mitigated this difference. There was a beneficial effect of communal culture in small volumes, and supplementation with a trophic ligand (Paf) further enhanced growth rates. For hybrid strain zygotes (B6CBF1) communal culture in KSOM media supplemented with amino acids, albumin and Paf under 5% O₂ resulted in complete rescue of their rate of accumulation of cells and blastocyst formation. Inbred strain (C57BL6/J) zygotes, however, still showed some retardation of development under these conditions. The additional supplementation of media with another trophic ligand (IGF1) showed a further additive beneficial effect on development of inbred strain embryos but they still showed a growth deficit of ~ 23% cell number. The results show that optimising the interactions between a range of culture conditions and media design can rescue hybrid strain embryos from a retarded rate of cell proliferation caused by culture in vitro, but this was incomplete for the B6 strain.
Conclusions: The results indicate that the growth requirement of embryos in vitro varies depending upon their genetic background and provide models for the further genetic analysis of embryo growth.
Figures
Similar articles
-
Evidence for the requirement of autocrine growth factors for development of mouse preimplantation embryos in vitro.Biol Reprod. 1997 Jan;56(1):229-37. doi: 10.1095/biolreprod56.1.229. Biol Reprod. 1997. PMID: 9002654
-
Culture of zygotes increases TRP53 [corrected] expression in B6 mouse embryos, which reduces embryo viability.Biol Reprod. 2007 Mar;76(3):362-7. doi: 10.1095/biolreprod.106.056838. Epub 2006 Nov 8. Biol Reprod. 2007. PMID: 17093197
-
Effect of retinoids and growth factor on in vitro bovine embryos produced under chemically defined conditions.Anim Reprod Sci. 2006 Oct;95(3-4):184-92. doi: 10.1016/j.anireprosci.2005.08.013. Epub 2005 Nov 14. Anim Reprod Sci. 2006. PMID: 16289874
-
Media composition: salts and osmolality.Methods Mol Biol. 2012;912:61-80. doi: 10.1007/978-1-61779-971-6_5. Methods Mol Biol. 2012. PMID: 22829369 Review.
-
Metabolic and developmental responses of preimplantation embryos to platelet activating factor (PAF).Reprod Fertil Dev. 1992;4(4):387-98. doi: 10.1071/rd9920387. Reprod Fertil Dev. 1992. PMID: 1461990 Review.
Cited by
-
Multiple facilitated glucose transporters SLC2As are required for normal mouse preimplantation embryo development.Am J Transl Res. 2019 Jun 15;11(6):3412-3425. eCollection 2019. Am J Transl Res. 2019. PMID: 31312354 Free PMC article.
-
Exogenous growth factors do not affect the development of individually cultured murine embryos.J Assist Reprod Genet. 2018 Mar;35(3):523-531. doi: 10.1007/s10815-017-1103-3. Epub 2017 Dec 21. J Assist Reprod Genet. 2018. PMID: 29270871 Free PMC article.
-
Cell-free fat extract improves embryo development and clinical outcomes in older women with previous in-vitro fertilization failure.Reprod Biol Endocrinol. 2025 Jan 17;23(1):8. doi: 10.1186/s12958-024-01341-4. Reprod Biol Endocrinol. 2025. PMID: 39825439 Free PMC article.
-
Dynamics of Mitochondrial DNA Copy Number and Membrane Potential in Mouse Pre-Implantation Embryos: Responses to Diverse Types of Oxidative Stress.Genes (Basel). 2024 Mar 16;15(3):367. doi: 10.3390/genes15030367. Genes (Basel). 2024. PMID: 38540426 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

