Targeting of erbB3 receptor to overcome resistance in cancer treatment
- PMID: 24886126
- PMCID: PMC4022415
- DOI: 10.1186/1476-4598-13-105
Targeting of erbB3 receptor to overcome resistance in cancer treatment
Abstract
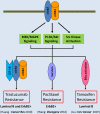
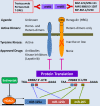
The erbB receptors, including the epidermal growth factor receptor (EGFR), erbB2 (also known as HER2/neu), erbB3 (or HER3), and erbB4 (or HER4), are often aberrantly activated in a wide variety of human cancers. They are excellent targets for selective anti-cancer therapies because of their transmembrane location and pro-oncogenic activity. While several therapeutic agents against erbB2 and/or EGFR have been used in the treatment of human cancers with efficacy, there has been relatively less emphasis on erbB3 as a molecular target. Elevated expression of erbB3 is frequently observed in various malignancies, where it promotes tumor progression via interactions with other receptor tyrosine kinases (RTKs) due to its lack of or weak intrinsic kinase activity. Studies on the underlying mechanisms implicate erbB3 as a major cause of treatment failure in cancer therapy, mainly through activation of the PI-3 K/Akt, MEK/MAPK, and Jak/Stat signaling pathways as well as Src kinase. It is believed that inhibition of erbB3 signaling may be required to overcome therapeutic resistance and effectively treat cancers. To date, no erbB3-targeted therapy has been approved for cancer treatment. Targeting of erbB3 receptor with a monoclonal antibody (Ab) is the only strategy currently under preclinical study and clinical evaluation. In this review, we focus on the role of erbB3-initiated signaling in the development of cancer drug resistance and discuss the latest advances in identifying therapeutic strategies inactivating erbB3 to overcome the resistance and enhance efficacy of cancer therapeutics.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

