Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS
- PMID: 24886384
- PMCID: PMC4038111
- DOI: 10.1186/1742-4690-11-35
Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS
Abstract
Background: Human immunodeficiency virus type 1(HIV-1) infects and activates innate immune cells in the brain resulting in inflammation and neuronal death with accompanying neurological deficits. Induction of inflammasomes causes cleavage and release of IL-1β and IL-18, representing pathogenic processes that underlie inflammatory diseases although their contribution HIV-associated brain disease is unknown.
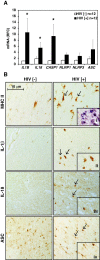
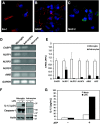
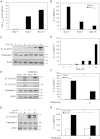
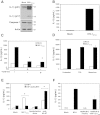
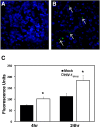
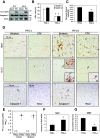
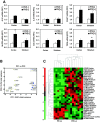
Results: Investigation of inflammasome-associated genes revealed that IL-1β, IL-18 and caspase-1 were induced in brains of HIV-infected persons and detected in brain microglial cells. HIV-1 infection induced pro-IL-1β in human microglia at 4 hr post-infection with peak IL-1β release at 24 hr, which was accompanied by intracellular ASC translocation and caspase-1 activation. HIV-dependent release of IL-1β from a human macrophage cell line, THP-1, was inhibited by NLRP3 deficiency and high extracellular [K+]. Exposure of microglia to HIV-1 gp120 caused IL-1β production and similarly, HIV-1 envelope pseudotyped viral particles induced IL-1β release, unlike VSV-G pseudotyped particles. Infection of cultured feline macrophages by the related lentivirus, feline immunodeficiency virus (FIV), also resulted in the prompt induction of IL-1β. In vivo FIV infection activated multiple inflammasome-associated genes in microglia, which was accompanied by neuronal loss in cerebral cortex and neurological deficits. Multivariate analyses of data from FIV-infected and uninfected animals disclosed that IL-1β, NLRP3 and caspase-1 expression in cerebral cortex represented key molecular determinants of neurological deficits.
Conclusions: NLRP3 inflammasome activation was an early and integral aspect of lentivirus infection of microglia, which was associated with lentivirus-induced brain disease. Inflammasome activation in the brain might represent a potential target for therapeutic interventions in HIV/AIDS.
Figures

Similar articles
-
HIV-1 Viral Protein R Activates NLRP3 Inflammasome in Microglia: implications for HIV-1 Associated Neuroinflammation.J Neuroimmune Pharmacol. 2017 Jun;12(2):233-248. doi: 10.1007/s11481-016-9708-3. Epub 2016 Oct 10. J Neuroimmune Pharmacol. 2017. PMID: 27726055
-
Sendai Virus V Protein Inhibits the Secretion of Interleukin-1β by Preventing NLRP3 Inflammasome Assembly.J Virol. 2018 Sep 12;92(19):e00842-18. doi: 10.1128/JVI.00842-18. Print 2018 Oct 1. J Virol. 2018. PMID: 30021903 Free PMC article.
-
HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood.PLoS One. 2018 Apr 19;13(4):e0192845. doi: 10.1371/journal.pone.0192845. eCollection 2018. PLoS One. 2018. PMID: 29672590 Free PMC article.
-
Neurologic disease in feline immunodeficiency virus infection: disease mechanisms and therapeutic interventions for NeuroAIDS.J Neurovirol. 2018 Apr;24(2):220-228. doi: 10.1007/s13365-017-0593-1. Epub 2017 Dec 15. J Neurovirol. 2018. PMID: 29247305 Review.
-
P2Y13 receptor involved in HIV-1 gp120 induced neuropathy in superior cervical ganglia through NLRP3 inflammasome activation.Neuropharmacology. 2024 Mar 1;245:109818. doi: 10.1016/j.neuropharm.2023.109818. Epub 2023 Dec 22. Neuropharmacology. 2024. PMID: 38142931 Review.
Cited by
-
Dopaminergic impact of cART and anti-depressants on HIV neuropathogenesis in older adults.Brain Res. 2019 Nov 15;1723:146398. doi: 10.1016/j.brainres.2019.146398. Epub 2019 Aug 21. Brain Res. 2019. PMID: 31442412 Free PMC article. Review.
-
Anti-inflammatory role of GM1 and other gangliosides on microglia.J Neuroinflammation. 2022 Jan 6;19(1):9. doi: 10.1186/s12974-021-02374-x. J Neuroinflammation. 2022. PMID: 34991625 Free PMC article.
-
HIV gp120 upregulates tonic inhibition through α5-containing GABAARs.Neuropharmacology. 2019 May 1;149:161-168. doi: 10.1016/j.neuropharm.2019.02.024. Epub 2019 Feb 20. Neuropharmacology. 2019. PMID: 30797029 Free PMC article.
-
Cognitive Burden of Common Non-antiretroviral Medications in HIV-Infected Women.J Acquir Immune Defic Syndr. 2018 Sep 1;79(1):83-91. doi: 10.1097/QAI.0000000000001755. J Acquir Immune Defic Syndr. 2018. PMID: 29781879 Free PMC article.
-
Methamphetamine and HIV-1 Tat Synergistically Induce Microglial Pyroptosis Via Activation of the AIM2 Inflammasome.Inflammation. 2025 Feb 19. doi: 10.1007/s10753-025-02266-9. Online ahead of print. Inflammation. 2025. PMID: 39969742
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

