Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden
- PMID: 24886434
- PMCID: PMC4025194
- DOI: 10.1186/1471-2407-14-317
Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumor burden
Abstract
Background: Current treatment of ovarian cancer patients with chemotherapy leaves behind a residual tumor which results in recurrent ovarian cancer within a short time frame. We have previously demonstrated that a single short-term treatment of ovarian cancer cells with chemotherapy in vitro resulted in a cancer stem cell (CSC)-like enriched residual population which generated significantly greater tumor burden compared to the tumor burden generated by control untreated cells. In this report we looked at the mechanisms of the enrichment of CSC-like residual cells in response to paclitaxel treatment.
Methods: The mechanism of survival of paclitaxel-treated residual cells at a growth inhibitory concentration of 50% (GI50) was determined on isolated tumor cells from the ascites of recurrent ovarian cancer patients and HEY ovarian cancer cell line by in vitro assays and in a mouse xenograft model.
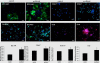
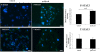
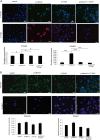
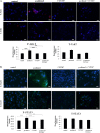
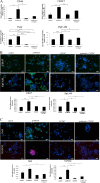
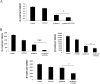
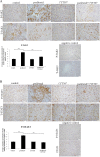
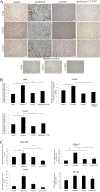
Results: Treatment of isolated tumor cells from the ascites of ovarian cancer patients and HEY ovarian cancer cell line with paclitaxel resulted in a CSC-like residual population which coincided with the activation of Janus activated kinase 2 (JAK2) and signal transducer and activation of transcription 3 (STAT3) pathway in paclitaxel surviving cells. Both paclitaxel-induced JAK2/STAT3 activation and CSC-like characteristics were inhibited by a low dose JAK2-specific small molecule inhibitor CYT387 (1 μM) in vitro. Subsequent, in vivo transplantation of paclitaxel and CYT387-treated HEY cells in mice resulted in a significantly reduced tumor burden compared to that seen with paclitaxel only-treated transplanted cells. In vitro analysis of tumor xenografts at protein and mRNA levels demonstrated a loss of CSC-like markers and CA125 expression in paclitaxel and CYT387-treated cell-derived xenografts, compared to paclitaxel only-treated cell-derived xenografts. These results were consistent with significantly reduced activation of JAK2 and STAT3 in paclitaxel and CYT387-treated cell-derived xenografts compared to paclitaxel only-treated cell derived xenografts.
Conclusions: This proof of principle study demonstrates that inhibition of the JAK2/STAT3 pathway by the addition of CYT387 suppresses the 'stemness' profile in chemotherapy-treated residual cells in vitro, which is replicated in vivo, leading to a reduced tumor burden. These findings have important implications for ovarian cancer patients who are treated with taxane and/or platinum-based therapies.
Figures


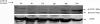

References
-
- Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3–S11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

