Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects
- PMID: 24886459
- PMCID: PMC4013295
- DOI: 10.1186/1750-1172-9-71
Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects
Abstract
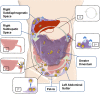
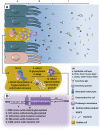
Pseudomyxoma peritonei (PMP, ORPHA26790) is a clinical syndrome characterized by progressive dissemination of mucinous tumors and mucinous ascites in the abdomen and pelvis. PMP is a rare disease with an estimated incidence of 1-2 out of a million. Clinically, PMP usually presents with a variety of unspecific signs and symptoms, including abdominal pain and distention, ascites or even bowel obstruction. It is also diagnosed incidentally at surgical or non-surgical investigations of the abdominopelvic viscera. PMP is a neoplastic disease originating from a primary mucinous tumor of the appendix with a distinctive pattern of the peritoneal spread. Computed tomography and histopathology are the most reliable diagnostic modalities. The differential diagnosis of the disease includes secondary peritoneal carcinomatoses and some rare peritoneal conditions. Optimal elimination of mucin and the mucin-secreting tumor comprises the current standard of care for PMP offered in specialized centers as visceral resections and peritonectomy combined with intraperitoneal chemotherapy. This multidisciplinary approach has reportedly provided a median survival rate of 16.3 years, a median progression-free survival rate of 8.2 years and 10- and 15-year survival rates of 63% and 59%, respectively. Despite its indolent, bland nature as a neoplasm, PMP is a debilitating condition that severely impacts quality of life. It tends to be diagnosed at advanced stages and frequently recurs after treatment. Being ignored in research, however, PMP remains a challenging, enigmatic entity. Clinicopathological features of the PMP syndrome and its morbid complications closely correspond with the multifocal distribution of the secreted mucin collections and mucin-secreting implants. Novel strategies are thus required to facilitate macroscopic, as well as microscopic, elimination of mucin and its source as the key components of the disease. In this regard, MUC2, MUC5AC and MUC5B have been found as the secreted mucins of relevance in PMP. Development of mucin-targeted therapies could be a promising avenue for future research which is addressed in this article.
Figures
References
-
- The portal for rare diseases and orphan drugs. [ http://www.orpha.net/consor/cgi-bin/index.php?lng=EN] - PMC - PubMed
-
- Werth R. Klinische und anatomische untersuchungen zur lehre von den bauchgeschwülsten und der laparatomie. Archiv Fur Gynakologie. 1884;24:100–118. doi: 10.1007/BF01837425. - DOI
-
- Fraenkel E. Ueber das sogennante pseudomyxoma perotonei. Munch Med Wochenschr. 1901;48:965–971.
-
- Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390–1408. doi: 10.1097/00000478-199512000-00006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

