Polymicrobial airway bacterial communities in adult bronchiectasis patients
- PMID: 24886473
- PMCID: PMC4031157
- DOI: 10.1186/1471-2180-14-130
Polymicrobial airway bacterial communities in adult bronchiectasis patients
Abstract
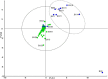
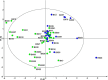
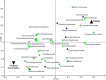
Background: Chronic airway infection contributes to the underlying pathogenesis of non-cystic fibrosis bronchiectasis (NCFBr). In contrast to other chronic airway infections, associated with COPD and CF bronchiectasis, where polymicrobial communities have been implicated in lung damage due to the vicious circle of recurrent bacterial infections and inflammation, there is sparse information on the composition of bacterial communities in NCFBr. Seventy consecutive patients were recruited from an outpatient adult NCFBr clinic. Bacterial communities in sputum samples were analysed by culture and pyrosequencing approaches. Bacterial sequences were analysed using partial least square discrimination analyses to investigate trends in community composition and identify those taxa that contribute most to community variation.
Results: The lower airway in NCFBr is dominated by three bacterial taxa Pasteurellaceae, Streptococcaceae and Pseudomonadaceae. Moreover, the bacterial community is much more diverse than indicated by culture and contains significant numbers of other genera including anaerobic Prevotellaceae, Veillonellaceae and Actinomycetaceae. We found particular taxa are correlated with different clinical states, 27 taxa were associated with acute exacerbations, whereas 11 taxa correlated with stable clinical states. We were unable to demonstrate a significant effect of antibiotic therapy, gender, or lung function on the diversity of the bacterial community. However, presence of clinically significant culturable taxa; particularly Pseudomonas aeruginosa and Haemophilus influenzae correlated with a significant change in the diversity of the bacterial community in the lung.
Conclusions: We have demonstrated that acute exacerbations, the frequency of exacerbation and episodes of clinical stability are correlated, in some patients, with a significantly different bacterial community structure, that are associated with a presence of particular taxa in the NCFBr lung. Moreover, there appears to be an inverse relationship between the abundance of P. aeruginosa and that of of H. influenzae within the NCFBr lung bacterial community. This interaction requires further exploration.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

