CA15-3 is a useful serum tumor marker for diagnostic integration of hybrid positron emission tomography with integrated computed tomography during follow-up of breast cancer patients
- PMID: 24886519
- PMCID: PMC4038066
- DOI: 10.1186/1471-2407-14-356
CA15-3 is a useful serum tumor marker for diagnostic integration of hybrid positron emission tomography with integrated computed tomography during follow-up of breast cancer patients
Abstract
Background: The aim of this study was to evaluate the value of CA15-3 for the diagnostic integration of molecular imaging findings performed with hybrid positron emission tomography and computed tomography (PETCT) technology.
Methods: We retrospectively selected 45 patients with a median age of 60 years (range 39-85 years) and a previous history of breast cancer (BC) who had already been treated with surgery and other treatments. Three measurements of CA15-3 were collected within 1 year before PETCT examination, at 6-9 months 3-6 months and 0-3 months before PETCT. The prolonged clinical outcome or imaging follow-up was used to define disease relapse. An increase in tumor marker value was compared with PETCT findings and disease relapse. Sensitivity and specificity for both tests were calculated with respect to clinical outcome.
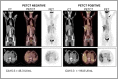
Results: Disease relapse was detected in 16 out of 45 BC patients. CA15-3 and PETCT showed 75% sensitivity with a specificity percentage of 76% for CA15-3 and 79% for PETCT. Serum CA15-3 expression levels were significantly higher in BC patients with multiple metastatic sites with hepatic involvement. Analysis of serial CA15-3 serum levels showed an increase in CA15-3 3-6 months before PETCT could identify BC patients at risk for relapse (AUC = 0.81). Moreover, patients receiving anti-hormonal or chemotherapy medications with negative PETCT and positive CA15-3 relapsed after a median time of 158 days compared to patients who were negative for both tests and who were free from disease for at least 1 year.
Conclusions: Our results showed that serial increases in CA15-3 can be used to predict positive PETCT results in BC patients during follow-up. Increased levels of CA15-3 may be considered an early warning sign in patients needing accurate molecular imaging investigations, as they are at higher risk of recurrence. In cases of elevated levels, multiple lesions or liver involvement may exist. Also, patients receiving chemotherapeutic or anti-hormonal treatment who have negative PETCT scans and increased CA15-3 serum levels should be considered at risk for relapse, because the CA15-3-linked biochemical signal of the presence of a tumor can predict positive metabolic imaging.
Figures

Similar articles
-
Could the serial determination of Ca15.3 serum improve the diagnostic accuracy of PET/CT?: results from small population with previous breast cancer.Ann Nucl Med. 2011 Aug;25(7):469-77. doi: 10.1007/s12149-011-0488-9. Epub 2011 Apr 8. Ann Nucl Med. 2011. PMID: 21476056
-
Correlation between cancer antigen 15.3 value and qualitative and semiquantitative parameters of positron emission tomography/computed tomography in breast cancer patients.Curr Radiopharm. 2014;7(1):20-8. doi: 10.2174/1874471007666140515111134. Curr Radiopharm. 2014. PMID: 24836946
-
[Elevation of serum Ca 15-3 antigen: an early indicator of distant metastasis from breast cancer. Retrospective analysis of 733 cases].Przegl Lek. 2001;58(6):498-503. Przegl Lek. 2001. PMID: 11816740 Polish.
-
Elevated serum CA15-3 levels correlate with positive estrogen receptor and initial favorable outcome in patients who died from recurrent breast cancer.Breast Cancer. 2003;10(3):220-7. doi: 10.1007/BF02966721. Breast Cancer. 2003. PMID: 12955034
-
Positron Emission Tomography Computed Tomography: A Guide for the General Radiologist.Can Assoc Radiol J. 2015 Nov;66(4):332-47. doi: 10.1016/j.carj.2015.02.003. Epub 2015 Aug 12. Can Assoc Radiol J. 2015. PMID: 26277234 Review.
Cited by
-
Evaluation of a newly developed quantitative determination kit for tumor marker CA15-3 with chemiluminescent assay.J Clin Lab Anal. 2018 Jan;32(1):e22158. doi: 10.1002/jcla.22158. Epub 2017 Feb 23. J Clin Lab Anal. 2018. PMID: 28233344 Free PMC article.
-
Clinical Indications and Impact on Management: Fourth and Subsequent Posttherapy Follow-up 18F-FDG PET/CT Scans in Oncology Patients.J Nucl Med. 2017 May;58(5):737-743. doi: 10.2967/jnumed.116.183111. Epub 2016 Nov 3. J Nucl Med. 2017. PMID: 27811123 Free PMC article.
-
Clinical utility of tumour marker velocity of cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in breast cancer surveillance.Breast. 2020 Aug;52:95-101. doi: 10.1016/j.breast.2020.05.005. Epub 2020 May 20. Breast. 2020. PMID: 32485607 Free PMC article.
-
Combining the tumor abnormal protein test with tests for carcinoembryonic antigens, cancer antigen 15-3, and/or cancer antigen 125 significantly increased their diagnostic sensitivity for breast cancer.Medicine (Baltimore). 2020 Jul 17;99(29):e21231. doi: 10.1097/MD.0000000000021231. Medicine (Baltimore). 2020. PMID: 32702897 Free PMC article.
-
CA27.29 as a tumour marker for risk evaluation and therapy monitoring in primary breast cancer patients.Tumour Biol. 2016 Oct;37(10):13769-13775. doi: 10.1007/s13277-016-5171-2. Epub 2016 Aug 1. Tumour Biol. 2016. PMID: 27481512 Clinical Trial.
References
-
- Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. on behalf of the ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl 7):vii11–vii19. - PubMed
-
- Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E. Correlation of high 18 F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–435. doi: 10.1007/s00259-010-1640-9. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

