MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress
- PMID: 24886523
- PMCID: PMC4046138
- DOI: 10.1186/1476-4598-13-129
MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress
Abstract
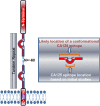
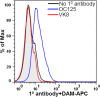
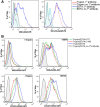
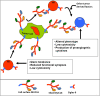
Over three decades have passed since the first report on the expression of CA125 by ovarian tumors. Since that time our understanding of ovarian cancer biology has changed significantly to the point that these tumors are now classified based on molecular phenotype and not purely on histological attributes. However, CA125 continues to be, with the recent exception of HE4, the only clinically reliable diagnostic marker for ovarian cancer. Many large-scale clinical trials have been conducted or are underway to determine potential use of serum CA125 levels as a screening modality or to distinguish between benign and malignant pelvic masses. CA125 is a peptide epitope of a 3-5 million Da mucin, MUC16. Here we provide an in-depth review of the literature to highlight the importance of CA125 as a prognostic and diagnostic marker for ovarian cancer. We focus on the increasing body of literature describing the biological role of MUC16 in the progression and metastasis of ovarian tumors. Finally, we consider previous and on-going efforts to develop therapeutic approaches to eradicate ovarian tumors by targeting MUC16. Even though CA125 is a crucial marker for ovarian cancer, the exact structural definition of this antigen continues to be elusive. The importance of MUC16/CA125 in the diagnosis, progression and therapy of ovarian cancer warrants the need for in-depth research on the biochemistry and biology of this mucin. A renewed focus on MUC16 is likely to culminate in novel and more efficient strategies for the detection and treatment of ovarian cancer.
Figures

References
-
- Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ, Haglund C, Harbeck N, Hayes DF, Holten-Andersen M, Klee GG, Lamerz R, Looijenga LH, Molina R, Nielsen HJ, Rittenhouse H, Semjonow A, Shih Ie M, Sibley P, Soletormos G, Stephan C, Sokoll L, Hoffman BR, Diamandis EP. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008;54:e11–e79. doi: 10.1373/clinchem.2008.105601. - DOI - PubMed
-
- Seltzer V, Drukker BH, Gillespie BW, Gossfeld LM, Grigsby PW, Harvey HA, Hendricks CB, Hummel S, Makuch RW, Monaco GP, Parham GP, Sawyers CL, West RJ, Alberts DS, Anderson B, Averette HE, Bast RC, Christian MC, Colombo N, Creasman WT, John P, Curtin JP, Gershenson DM, Hoskins WJ, Karlan BY, Kramer BS, Markman M, McGuire WP, Ozols RF, Pecorelli S. et al.NIH consensus conference: ovarian cancer: screening, treatment, and follow-up. NIH consensus development panel on ovarian cancer. JAMA. 1995;273:491–497. doi: 10.1001/jama.1995.03520300065039. - DOI - PubMed
-
- Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K, Vergote I, Cervantes A, Vermorken J. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004;96:487–488. - PubMed
-
- Vasudev NS, Trigonis I, Cairns DA, Hall GD, Jackson DP, Broadhead T, Buxton J, Hutson R, Nugent D, Perren TJ. The prognostic and predictive value of CA-125 regression during neoadjuvant chemotherapy for advanced ovarian or primary peritoneal carcinoma. Arch Gynecol Obstet. 2011;284:221–227. doi: 10.1007/s00404-010-1655-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

