Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons
- PMID: 24886596
- PMCID: PMC4036648
- DOI: 10.1186/1744-8069-10-31
Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons
Abstract
Background: Artemin (Artn), a member of the glial cell line-derived growth factor (GDNF) family, supports the development and function of a subpopulation of peptidergic, TRPV1-positive sensory neurons. Artn (enovin, neublastin) is elevated in inflamed tissue and its injection in skin causes transient thermal hyperalgesia. A genome wide expression analysis of trigeminal ganglia of mice that overexpress Artn in the skin (ART-OE mice) showed elevation in nicotinic acetylcholine receptor (nAChR) subunits, suggesting these ion channels contribute to Artn-induced sensitivity. Here we have used gene expression, immunolabeling, patch clamp electrophysiology and behavioral testing assays to investigate the link between Artn, nicotinic subunit expression and thermal hypersensitivity.
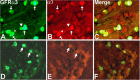
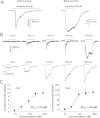
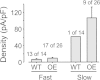
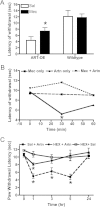
Results: Reverse transcriptase-PCR validation showed increased levels of mRNAs encoding the nAChR subunits α3 (13.3-fold), β3 (4-fold) and β4 (7.7-fold) in trigeminal ganglia and α3 (4-fold) and β4 (2.8-fold) in dorsal root ganglia (DRG) of ART-OE mice. Sensory ganglia of ART-OE mice had increased immunoreactivity for nAChRα3 and exhibited increased overlap in labeling with GFRα3-positive neurons. Patch clamp analysis of back-labeled cutaneous afferents showed that while the majority of nicotine-evoked currents in DRG neurons had biophysical and pharmacological properties of α7-subunit containing nAChRs, the Artn-induced increase in α3 and β4 subunits resulted in functional channels. Behavioral analysis of ART-OE and wildtype mice showed that Artn-induced thermal hyperalgesia can be blocked by mecamylamine or hexamethonium. Complete Freund's adjuvant (CFA) inflammation of paw skin, which causes an increase in Artn in the skin, also increased the level of nAChR mRNAs in DRG. Finally, the increase in nAChRs transcription was not dependent on the Artn-induced increase in TRPV1 or TRPA1 in ART-OE mice since nAChRs were elevated in ganglia of TRPV1/TRPA1 double knockout mice.
Conclusions: These findings suggest that Artn regulates the expression and composition of nAChRs in GFRα3 nociceptors and that these changes contribute to the thermal hypersensitivity that develops in response to Artn injection and perhaps to inflammation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

