Tobacco BY-2 cell-free lysate: an alternative and highly-productive plant-based in vitro translation system
- PMID: 24886601
- PMCID: PMC4101825
- DOI: 10.1186/1472-6750-14-37
Tobacco BY-2 cell-free lysate: an alternative and highly-productive plant-based in vitro translation system
Abstract
Background: Cell-free protein synthesis is a rapid and efficient method for the production of recombinant proteins. Usage of prokaryotic cell-free extracts often leads to non-functional proteins. Eukaryotic counterparts such as wheat germ extract (WGE) and rabbit reticulocyte lysate (RLL) may improve solubility and promote the correct folding of eukaryotic multi-domain proteins that are difficult to express in bacteria. However, the preparation of WGEs is complex and time-consuming, whereas RLLs suffer from low yields. Here we report the development of a novel cell-free system based on tobacco Bright Yellow 2 (BY-2) cells harvested in the exponential growth phase.
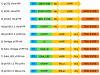
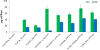
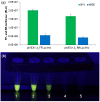
Results: The highly-productive BY-2 lysate (BYL) can be prepared quickly within 4-5 h, compared to 4-5 d for WGE. The efficiency of the BYL was tested using three model proteins: enhanced yellow fluorescent protein (eYFP) and two versions of luciferase. The added mRNA was optimized by testing different 5' and 3' untranslated regions (UTRs). The protein yield in batch and dialysis reactions using BYL was much higher than that of a commercial Promega WGE preparation, achieving a maximum yield of 80 μg/mL of eYFP and 100 μg/mL of luciferase, compared to only 45 μg/mL of eYFP and 35 μg/mL of luciferase in WGEs. In dialysis reactions, the BYL yielded about 400 μg/mL eYFP, representing up to 50% more of the target protein than the Promega WGE, and equivalent to the amount using 5Prime WGE system.
Conclusions: Due to the high yield and the short preparation time the BYL represents a remarkable improvement over current eukaryotic cell-free systems.
Figures




References
-
- Swartz JR. Transforming biochemical engineering with cell-free biology. AIChE Journal. 2012;58:5–13. doi: 10.1002/aic.13701. - DOI
-
- Kim HC, Kim TW, Kim DM. Prolonged production of proteins in a cell-free protein synthesis system using polymeric carbohydrates as an energy source. Process Biochem. 2011;46(6):1366–1369. doi: 10.1016/j.procbio.2011.03.008. - DOI
-
- Zawada JF, Yin G, Steiner AR, Yang J, Naresh A, Roy SM, Gold DS, Heinsohn HG, Murray CJ. Microscale to manufacturing scale-up of cell-free cytokine production–a new approach for shortening protein production development timelines. Biotechnol Bioeng. 2011;108(7):1570–1578. doi: 10.1002/bit.23103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

