C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment
- PMID: 24886617
- PMCID: PMC4076630
- DOI: 10.1186/bcr3665
C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment
Abstract
Introduction: Although C-X-C motif chemokine 12 (CXCL12) has been shown to bind to C-X-C chemokine receptor type 7 (CXCR7), the exact molecular mechanism regulations by CXCL12/CXCR7 axis in breast tumor growth and metastasis are not well understood. CXCR7 expression has been shown to be upregulated during pathological processes such as inflammation and cancer.
Methods: Breast cancer cell lines were genetically silenced or pharmacologically inhibited for CXCR7 and/or its downstream target signal transducer and activator of transcription 3 (STAT3). 4T1 or 4T1 downregulated for CXCR7 and 4T1.2 breast cancer cell lines were injected in mammary gland of BALB/c mice to form tumors, and the molecular pathways regulating tumor growth and metastasis were assessed.
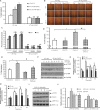
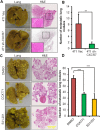
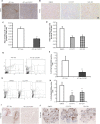
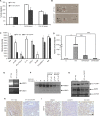
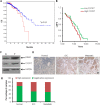
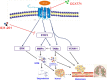
Results: In this study, we observed that CXCL12 enhances CXCR7-mediated breast cancer migration. Furthermore, genetic silencing or pharmacologic inhibition of CXCR7 reduced breast tumor growth and metastasis. Further elucidation of mechanisms revealed that CXCR7 mediates tumor growth and metastasis by activating proinflammatory STAT3 signaling and angiogenic markers. Furthermore, enhanced breast tumorigenicity and invasiveness were associated with macrophage infiltration. CXCR7 recruits tumor-promoting macrophages (M2) to the tumor site through regulation of the macrophage colony-stimulating factor (M-CSF)/macrophage colony-stimulating factor receptor (MCSF-R) signaling pathway. In addition, CXCR7 regulated breast cancer metastasis by enhancing expression of metalloproteinases (MMP-9, MMP-2) and vascular cell-adhesion molecule-1 (VCAM-1). We also observed that CXCR7 is highly expressed in invasive ductal carcinoma (IDC) and metastatic breast tissue in human patient samples. In addition, high CXCR7 expression in tumors correlates with worse prognosis for both overall survival and lung metastasis-free survival in IDC patients.
Conclusion: These observations reveal that CXCR7 enhances breast cancer growth and metastasis via a novel pathway by modulating the tumor microenvironment. These findings identify CXCR7-mediated STAT3 activation and modulation of the tumor microenvironment as novel regulation of breast cancer growth and metastasis. These studies indicate that new strategies using CXCR7 inhibitors could be developed for antimetastatic therapy.
Figures

References
-
- Kan N, Kuwata K, Mise K, Kodama H. Effective therapeutic regimens for patients with triple-negative (ER/PgR/HER2-negative) metastatic breast cancer. Gan To Kagaku Ryoho. 2010;37:1259–1264. - PubMed
-
- Fernandez Y, Cueva J, Palomo AG, Ramos M, de Juan A, Calvo L, Garcia-Mata J, Garcia-Teijido P, Pelaez I, Garcia-Estevez L. Novel therapeutic approaches to the treatment of metastatic breast cancer. Cancer Treat Rev. 2010;36:33–42. doi: 10.1016/j.ctrv.2009.10.001. doi:10.1016/j.ctrv.2009.10.001. - DOI - PubMed
-
- Nasser MW, Qamri Z, Deol YS, Smith D, Shilo K, Zou X, Ganju RK. Crosstalk between chemokine receptor CXCR4 and cannabinoid receptor CB2 in modulating breast cancer growth and invasion. PLoS One. 2011;6:e23901. doi: 10.1371/journal.pone.0023901. doi:10.1371/journal.pone.0023901PONE-D-11-12697. - DOI - PMC - PubMed
-
- Kang H, Mansel RE, Jiang WG. Genetic manipulation of stromal cell-derived factor-1 attests the pivotal role of the autocrine SDF-1-CXCR4 pathway in the aggressiveness of breast cancer cells. Int J Oncol. 2005;26:1429–1434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

