Immature dengue virus is infectious in human immature dendritic cells via interaction with the receptor molecule DC-SIGN
- PMID: 24886790
- PMCID: PMC4041791
- DOI: 10.1371/journal.pone.0098785
Immature dengue virus is infectious in human immature dendritic cells via interaction with the receptor molecule DC-SIGN
Abstract
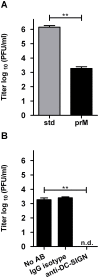
Background: Dengue Virus (DENV) is the most common mosquito-borne viral infection worldwide. Important target cells during DENV infection are macrophages, monocytes, and immature dendritic cells (imDCs). DENV-infected cells are known to secrete a large number of partially immature and fully immature particles alongside mature virions. Fully immature DENV particles are considered non-infectious, but antibodies have been shown to rescue their infectious properties. This suggests that immature DENV particles only contribute to the viral load observed in patients with a heterologous DENV re-infection.
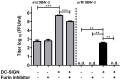
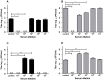
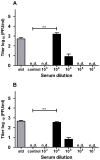
Methodology/principal findings: In this study, we re-evaluated the infectious properties of fully immature particles in absence and presence of anti-DENV human serum. We show that immature DENV is infectious in cells expressing DC-SIGN. Furthermore, we demonstrate that immature dendritic cells, in contrast to macrophage-like cells, do not support antibody-dependent enhancement of immature DENV.
Conclusions/significance: Our data shows that immature DENV can infect imDCs through interaction with DC-SIGN, suggesting that immature and partially immature DENV particles may contribute to dengue pathogenesis during primary infection. Furthermore, since antibodies do not further stimulate DENV infectivity on imDCs we propose that macrophages/monocytes rather than imDCs contribute to the increased viral load observed during severe heterotypic DENV re-infections.
Conflict of interest statement
Figures
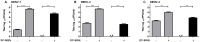
Similar articles
-
Beyond attachment: Roles of DC-SIGN in dengue virus infection.Traffic. 2017 Apr;18(4):218-231. doi: 10.1111/tra.12469. Epub 2017 Feb 28. Traffic. 2017. PMID: 28128492 Free PMC article.
-
Soluble isoforms of the DC-SIGN receptor can increase the dengue virus infection in immature dendritic cells.Braz J Infect Dis. 2024 Nov-Dec;28(6):103873. doi: 10.1016/j.bjid.2024.103873. Epub 2024 Sep 25. Braz J Infect Dis. 2024. PMID: 39341603 Free PMC article.
-
Crucial role of the N-glycans on the viral E-envelope glycoprotein in DC-SIGN-mediated dengue virus infection.Antiviral Res. 2012 Dec;96(3):280-7. doi: 10.1016/j.antiviral.2012.10.007. Epub 2012 Oct 31. Antiviral Res. 2012. PMID: 23124109
-
Early dengue virus interactions: the role of dendritic cells during infection.Virus Res. 2016 Sep 2;223:88-98. doi: 10.1016/j.virusres.2016.07.001. Epub 2016 Jul 2. Virus Res. 2016. PMID: 27381061 Review.
-
Dengue virus life cycle: viral and host factors modulating infectivity.Cell Mol Life Sci. 2010 Aug;67(16):2773-86. doi: 10.1007/s00018-010-0357-z. Epub 2010 Apr 6. Cell Mol Life Sci. 2010. PMID: 20372965 Free PMC article. Review.
Cited by
-
TLR2 axis on peripheral blood mononuclear cells regulates inflammatory responses to non-infectious immature dengue virus particles.PLoS Pathog. 2022 Oct 14;18(10):e1010499. doi: 10.1371/journal.ppat.1010499. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36240261 Free PMC article.
-
Transcriptional Changes during Naturally Acquired Zika Virus Infection Render Dendritic Cells Highly Conducive to Viral Replication.Cell Rep. 2017 Dec 19;21(12):3471-3482. doi: 10.1016/j.celrep.2017.11.087. Cell Rep. 2017. PMID: 29262327 Free PMC article.
-
Beyond attachment: Roles of DC-SIGN in dengue virus infection.Traffic. 2017 Apr;18(4):218-231. doi: 10.1111/tra.12469. Epub 2017 Feb 28. Traffic. 2017. PMID: 28128492 Free PMC article.
-
Different Associations between DC-SIGN Promoter-336G/A (rs4804803) Polymorphism with Severe Dengue in Asians and South-Central Americans: a Meta-Analysis.Int J Environ Res Public Health. 2019 Apr 25;16(8):1475. doi: 10.3390/ijerph16081475. Int J Environ Res Public Health. 2019. PMID: 31027310 Free PMC article.
-
A novel mechanism of antibody-mediated enhancement of flavivirus infection.PLoS Pathog. 2017 Sep 15;13(9):e1006643. doi: 10.1371/journal.ppat.1006643. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28915259 Free PMC article.
References
-
- World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases (TDR) (2009) Dengue: Guidelines for diagnosis, treatment, prevention and control. Geneva.
-
- Halstead SB (2007) Dengue. The Lancet 370: 1644–1652. - PubMed
-
- Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60: 421–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

