NOD-like receptors in intestinal homeostasis and epithelial tissue repair
- PMID: 24886810
- PMCID: PMC4100112
- DOI: 10.3390/ijms15069594
NOD-like receptors in intestinal homeostasis and epithelial tissue repair
Abstract
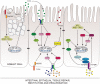
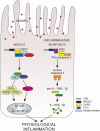
The intestinal epithelium constitutes a dynamic physical barrier segregating the luminal content from the underlying mucosal tissue. Following injury, the epithelial integrity is restored by rapid migration of intestinal epithelial cells (IECs) across the denuded area in a process known as wound healing. Hence, through a sequence of events involving restitution, proliferation and differentiation of IECs the gap is resealed and homeostasis reestablished. Relapsing damage followed by healing of the inflamed mucosa is a hallmark of several intestinal disorders including inflammatory bowel diseases (IBD). While several regulatory peptides, growth factors and cytokines stimulate restitution of the epithelial layer after injury, recent evidence in the field underscores the contribution of innate immunity in controlling this process. In particular, nucleotide-binding and oligomerization domain-like receptors (NLRs) play critical roles in sensing the commensal microbiota, maintaining homeostasis, and regulating intestinal inflammation. Here, we review the process of intestinal epithelial tissue repair and we specifically focus on the impact of NLR-mediated signaling mechanisms involved in governing epithelial wound healing during disease.
Figures
Similar articles
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Expression and functional importance of innate immune receptors by intestinal epithelial cells.Cell Mol Life Sci. 2011 Nov;68(22):3661-73. doi: 10.1007/s00018-011-0829-9. Epub 2011 Oct 8. Cell Mol Life Sci. 2011. PMID: 21984599 Free PMC article. Review.
-
Epithelial restitution and wound healing in inflammatory bowel disease.World J Gastroenterol. 2008 Jan 21;14(3):348-53. doi: 10.3748/wjg.14.348. World J Gastroenterol. 2008. PMID: 18200658 Free PMC article. Review.
-
Wound healing of intestinal epithelial cells.World J Gastroenterol. 2011 May 7;17(17):2161-71. doi: 10.3748/wjg.v17.i17.2161. World J Gastroenterol. 2011. PMID: 21633524 Free PMC article. Review.
-
NOD-Like Receptors: Guardians of Intestinal Mucosal Barriers.Physiology (Bethesda). 2015 May;30(3):241-50. doi: 10.1152/physiol.00025.2014. Physiology (Bethesda). 2015. PMID: 25933824 Review.
Cited by
-
Vibrio vulnificus VvpE inhibits mucin 2 expression by hypermethylation via lipid raft-mediated ROS signaling in intestinal epithelial cells.Cell Death Dis. 2015 Jun 18;6(6):e1787. doi: 10.1038/cddis.2015.152. Cell Death Dis. 2015. PMID: 26086960 Free PMC article.
-
Bacillus coagulans TL3 Inhibits LPS-Induced Caecum Damage in Rat by Regulating the TLR4/MyD88/NF-κB and Nrf2 Signal Pathways and Modulating Intestinal Microflora.Oxid Med Cell Longev. 2022 Feb 7;2022:5463290. doi: 10.1155/2022/5463290. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35178157 Free PMC article.
-
Nucleotide-mediated SPDEF modulates TFF3-mediated wound healing and intestinal barrier function during the weaning process.Sci Rep. 2018 Mar 19;8(1):4827. doi: 10.1038/s41598-018-23218-4. Sci Rep. 2018. PMID: 29555969 Free PMC article.
-
Glycine Relieves Intestinal Injury by Maintaining mTOR Signaling and Suppressing AMPK, TLR4, and NOD Signaling in Weaned Piglets after Lipopolysaccharide Challenge.Int J Mol Sci. 2018 Jul 6;19(7):1980. doi: 10.3390/ijms19071980. Int J Mol Sci. 2018. PMID: 29986455 Free PMC article.
-
The Role of Aeromonas-Goblet Cell Interactions in Melatonin-Mediated Improvements in Sleep Deprivation-Induced Colitis.Oxid Med Cell Longev. 2022 Mar 20;2022:8133310. doi: 10.1155/2022/8133310. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35355860 Free PMC article.
References
-
- Long M.D., Hutfless S., Kappelman M.D., Khalili H., Kaplan G.G., Bernstein C.N., Colombel J.F., Gower-Rousseau C., Herrinton L., Velayos F., et al. Challenges in designing a national surveillance program for inflammatory bowel disease in the united states. Inflamm. Bowel Dis. 2014;20:398–415. doi: 10.1097/01.MIB.0000435441.30107.8b. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

