β-lactam antibiotic concentrations during continuous renal replacement therapy
- PMID: 24886826
- PMCID: PMC4075122
- DOI: 10.1186/cc13886
β-lactam antibiotic concentrations during continuous renal replacement therapy
Abstract
Introduction: The use of standard doses of β-lactam antibiotics during continuous renal replacement therapy (CRRT) may result in inadequate serum concentrations. The aim of this study was to evaluate the adequacy of unadjusted drug regimens (i.e., similar to those used in patients with normal renal function) in patients treated with CRRT and the influence of CRRT intensity on drug clearance.
Methods: We reviewed data from 50 consecutive adult patients admitted to our Department of Intensive Care in whom routine therapeutic drug monitoring (TDM) of broad-spectrum β-lactam antibiotics (ceftazidime or cefepime, CEF; piperacillin/tazobactam; TZP; meropenem, MEM) was performed using unadjusted β-lactam antibiotics regimens (CEF = 2 g q8h; TZP = 4 g q6h; MEM = 1 g q8h). Serum drug concentrations were measured twice during the elimination phase by high-performance liquid chromatography (HPLC-UV). We considered therapy was adequate when serum drug concentrations were between 4 and 8 times the minimal inhibitory concentration (MIC) of Pseudomonas aeruginosa during optimal periods of time for each drug (≥70% for CEF; ≥ 50% for TZP; ≥ 40% for MEM). Therapy was considered as early (ET) or late (LT) phase if TDM was performed within 48 hours of antibiotic initiation or later on, respectively.
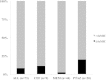
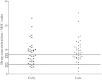
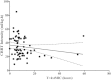
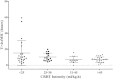
Results: We collected 73 serum samples from 50 patients (age 58 ± 13 years; Acute Physiology and Chronic Health Evaluation II (APACHE II) score on admission 21 (17-25)), 35 during ET and 38 during LT. Drug concentrations were above 4 times the MIC in 63 (90%), but above 8 times the MIC in 39 (53%) samples. The proportions of patients with adequate drug concentrations during ET and LT were quite similar. We found a weak but significant correlation between β-lactam antibiotics clearance and CRRT intensity.
Conclusions: In septic patients undergoing CRRT, doses of β-lactam antibiotics similar to those given to patients with normal renal function achieved drug levels above the target threshold in 90% of samples. Nevertheless, 53% of samples were associated with very high drug levels and daily drug regimens may need to be adapted accordingly.
Figures


Comment in
-
Beta-lactam antibiotic dosing during continuous renal replacement therapy: how can we optimize therapy?Crit Care. 2014 Jun 26;18(3):158. doi: 10.1186/cc13945. Crit Care. 2014. PMID: 25043643 Free PMC article.
-
Acute kidney injury: Antibiotic therapy during CRRT--getting the dose just right.Nat Rev Nephrol. 2014 Sep;10(9):486-8. doi: 10.1038/nrneph.2014.136. Epub 2014 Jul 29. Nat Rev Nephrol. 2014. PMID: 25072121 No abstract available.
References
-
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y, Reinhart K, EPIC II. Group of Investigators. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. - DOI - PubMed
-
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. - DOI - PubMed
-
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

