Functional effects of Toll-like receptor (TLR)3, 7, 9, RIG-I and MDA-5 stimulation in nasal epithelial cells
- PMID: 24886842
- PMCID: PMC4041746
- DOI: 10.1371/journal.pone.0098239
Functional effects of Toll-like receptor (TLR)3, 7, 9, RIG-I and MDA-5 stimulation in nasal epithelial cells
Abstract
Background: The human nasal epithelium is an important physical barrier, and a part of the innate immune defense that protect against pathogens. The epithelial cells recognize microbial components by pattern-recognition receptors (PRRs), and thereby trigger an immune response. Even though TLR3, TLR7, TLR9, RIG-I and MDA-5 are all known to respond to viral stimulation, their potential role in chronic airway inflammation triggered by local cytokine release remains to be established.
Methods: mRNA and corresponding protein expression of TLR3, TLR7, TLR9, RIG-I and MDA-5 were analyzed in nasal biopsies and various upper airway epithelial cell lines using real-time reverse transcription PCR, immunohistochemistry and flow cytometry. Ligand induced, cytokine release, was evaluated with ELISA.
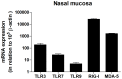
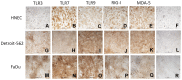
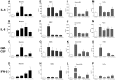
Results: Nasal biopsies were found to express TLR3, TLR7, TLR9, RIG-I and MDA-5, with the most abundant expression in the surface epithelium. These receptors were verified in primary human nasal epithelial cell (HNEC) as well as in the airway epithelial cell lines Detroit-562 and FaDu. Poly(I:C) (TLR3) and R-837 (TLR7) stimulation increased secretion of IL-6 and GM-CSF from the nasal mucosa and the epithelial cell lines. CpG (TLR9) stimulation caused release of IL-8 in the nasal mucosa and in FaDu. Poly(I:C)/LyoVec (RIG-I/MDA-5) stimulation activated the secretion of IFN-β in the nasal mucosa. A corresponding release was also detected from HNEC and Detroit-562.
Conclusion: The nasal epithelium has the ability to recognize viral intrusion through TLR and RLR receptors, and the subsequent response might have a role in exacerbation of inflammatory diseases like allergic rhinitis and chronic rhinosinusitis.
Conflict of interest statement
Figures

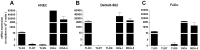
References
-
- Holgate ST (2007) Epithelium dysfunction in asthma. Journal of Allergy and Clinical Immunology 120: 1233–1246. - PubMed
-
- Bals R, Hiemstra PS (2004) Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. European Respiratory Journal 23: 327–333. - PubMed
-
- Kawai T, Akira S (2006) TLR signaling. Cell Death and Differentiation 13: 816–825. - PubMed
-
- Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140: 805–820. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

