The preclinical Alzheimer cognitive composite: measuring amyloid-related decline
- PMID: 24886908
- PMCID: PMC4439182
- DOI: 10.1001/jamaneurol.2014.803
The preclinical Alzheimer cognitive composite: measuring amyloid-related decline
Abstract
Importance: As Alzheimer disease (AD) research moves to intervene in presymptomatic phases of the disease, we must develop outcome measures sensitive to the earliest disease-related changes.
Objective: To demonstrate the feasibility of a cognitive composite outcome for clinically normal elderly participants with evidence of AD pathology using the ADCS Preclinical Alzheimer Cognitive Composite (ADCS-PACC). The ADCS-PACC combines tests that assess episodic memory, timed executive function, and global cognition. The ADCS-PACC is the primary outcome measure for the first clinical trial in preclinical AD (ie, the Anti-Amyloid Treatment in Asymptomatic Alzheimer's study).
Design, setting, and participants: With the ADCS-PACC, we derive pilot estimates of amyloid-related decline using data from 2 observational studies conducted in North America and another conducted in Australia. The participants analyzed had normal cognition and mean ages of 75.81, 71.37, and 79.42 years across the 3 studies.
Main outcomes and measures: For the 2 studies that collected data on Aβ levels (ADNI and AIBL), we estimate decline in a preclinical AD "Aβ-positive" placebo group and compare them with an "Aβ-negative" group. For the study that did not include data on Aβ levels (the ADCS Prevention Instrument [ADCS-PI] study), we grouped participants by the presence of APOE-ε4 and by clinical progression.
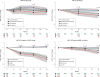
Results: In ADNI, Aβ-positive participants showed more decline than did Aβ-negative participants with regard to the ADCS-PACC score at 24 months (mean [SE] difference, -1.239 [0.522] [95% CI, -2.263 to -0.215]; P = .02). In AIBL, the mean (SE) difference is significant at both 18 months (-1.009 [0.406] [95% CI, -1.805 to -0.213]; P = .01) and 36 months (-1.404 [0.452] [95% CI, -2.290 to -0.519]; P = .002). In the ADCS-PI study, APOE-ε4 allele carriers performed significantly worse on the ADCS-PACC at 24 months (mean [SE] score, -0.742 [0.294] [95% CI, -1.318 to -0.165]; P = .01) and 36 months (-1.531 [0.469] [95% CI, -2.450 to -0.612]; P = .001). In the ADCS-PI study, cognitively normal participants who progress from a global Clinical Dementia Rating score of 0 are significantly worse on the ADCS-PACC than cognitively normal participants who are stable with a global Clinical Dementia Rating score of 0 at months 12, 24, and 36 (mean [SE] ADCS-PACC score, -4.471 [0.702] [95% CI, -5.848 to -3.094]; P < .001). Using pilot estimates of variance and assuming 500 participants per group with 30% attrition and a 5% α level, we project 80% power to detect effects in the range of Δ = 0.467 to 0.733 on the ADCS-PACC.
Conclusions and relevance: Analyses of at-risk cognitively normal populations suggest that we can reliably measure the first signs of cognitive decline with the ADCS-PACC. These analyses also suggest the feasibility of secondary prevention trials.
Conflict of interest statement
Figures
Comment in
-
Secondary prevention trials in Alzheimer disease: the challenge of identifying a meaningful end point.JAMA Neurol. 2014 Aug;71(8):947-9. doi: 10.1001/jamaneurol.2014.1120. JAMA Neurol. 2014. PMID: 24886838 Free PMC article. No abstract available.
References
-
- Villemagne VL, Burnham S, Bourgeat P, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. - PubMed
-
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

