Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation
- PMID: 24886962
- PMCID: PMC4271840
- DOI: 10.1016/j.yexmp.2014.05.009
Pentoxifylline attenuates nitrogen mustard-induced acute lung injury, oxidative stress and inflammation
Abstract
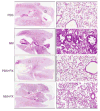
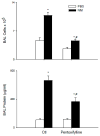
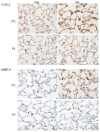
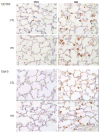
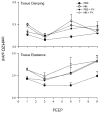
Nitrogen mustard (NM) is a toxic alkylating agent that causes damage to the respiratory tract. Evidence suggests that macrophages and inflammatory mediators including tumor necrosis factor (TNF)α contribute to pulmonary injury. Pentoxifylline is a TNFα inhibitor known to suppress inflammation. In these studies, we analyzed the ability of pentoxifylline to mitigate NM-induced lung injury and inflammation. Exposure of male Wistar rats (150-174 g; 8-10 weeks) to NM (0.125 mg/kg, i.t.) resulted in severe histopathological changes in the lung within 3d of exposure, along with increases in bronchoalveolar lavage (BAL) cell number and protein, indicating inflammation and alveolar-epithelial barrier dysfunction. This was associated with increases in oxidative stress proteins including lipocalin (Lcn)2 and heme oxygenase (HO)-1 in the lung, along with pro-inflammatory/cytotoxic (COX-2(+) and MMP-9(+)), and anti-inflammatory/wound repair (CD163+ and Gal-3(+)) macrophages. Treatment of rats with pentoxifylline (46.7 mg/kg, i.p.) daily for 3d beginning 15 min after NM significantly reduced NM-induced lung injury, inflammation, and oxidative stress, as measured histologically and by decreases in BAL cell and protein content, and levels of HO-1 and Lcn2. Macrophages expressing COX-2 and MMP-9 also decreased after pentoxifylline, while CD163+ and Gal-3(+) macrophages increased. This was correlated with persistent upregulation of markers of wound repair including pro-surfactant protein-C and proliferating nuclear cell antigen by Type II cells. NM-induced lung injury and inflammation were associated with alterations in the elastic properties of the lung, however these were largely unaltered by pentoxifylline. These data suggest that pentoxifylline may be useful in treating acute lung injury, inflammation and oxidative stress induced by vesicants.
Keywords: Lung injury; Macrophages; Mustards; Oxidative stress; Pentoxifylline; Vesicants.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors do not have any conflict of interest.
Figures



Similar articles
-
Role of macrophage bioenergetics in N-acetylcysteine-mediated mitigation of lung injury and oxidative stress induced by nitrogen mustard.Toxicol Appl Pharmacol. 2024 Apr;485:116908. doi: 10.1016/j.taap.2024.116908. Epub 2024 Mar 19. Toxicol Appl Pharmacol. 2024. PMID: 38513841 Free PMC article.
-
Attenuation of Nitrogen Mustard-Induced Pulmonary Injury and Fibrosis by Anti-Tumor Necrosis Factor-α Antibody.Toxicol Sci. 2015 Nov;148(1):71-88. doi: 10.1093/toxsci/kfv161. Epub 2015 Aug 4. Toxicol Sci. 2015. PMID: 26243812 Free PMC article.
-
Lung injury, oxidative stress and fibrosis in mice following exposure to nitrogen mustard.Toxicol Appl Pharmacol. 2020 Jan 15;387:114798. doi: 10.1016/j.taap.2019.114798. Epub 2019 Oct 31. Toxicol Appl Pharmacol. 2020. PMID: 31678244 Free PMC article.
-
Inflammatory mechanisms of pulmonary injury induced by mustards.Toxicol Lett. 2016 Feb 26;244:2-7. doi: 10.1016/j.toxlet.2015.10.011. Epub 2015 Oct 23. Toxicol Lett. 2016. PMID: 26478570 Free PMC article. Review.
-
Targeting Tumor Necrosis Factor Alpha to Mitigate Lung Injury Induced by Mustard Vesicants and Radiation.Disaster Med Public Health Prep. 2023 Oct 18;17:e553. doi: 10.1017/dmp.2023.178. Disaster Med Public Health Prep. 2023. PMID: 37848400 Free PMC article. Review.
Cited by
-
The interplay of DAMPs, TLR4, and proinflammatory cytokines in pulmonary fibrosis.J Mol Med (Berl). 2021 Oct;99(10):1373-1384. doi: 10.1007/s00109-021-02113-y. Epub 2021 Jul 13. J Mol Med (Berl). 2021. PMID: 34258628 Free PMC article. Review.
-
Assessment of mustard vesicant lung injury and anti-TNF-α efficacy in rodents using live-animal imaging.Ann N Y Acad Sci. 2020 Nov;1480(1):246-256. doi: 10.1111/nyas.14525. Epub 2020 Nov 9. Ann N Y Acad Sci. 2020. PMID: 33165947 Free PMC article.
-
Nitrogen mustard hydrochloride-induced acute respiratory failure and myelosuppression: A case report.Exp Ther Med. 2015 Oct;10(4):1293-1296. doi: 10.3892/etm.2015.2664. Epub 2015 Jul 29. Exp Ther Med. 2015. PMID: 26622480 Free PMC article.
-
Recent Pathophysiological Aspects of Peyronie's Disease: Role of Free Radicals, Rationale, and Therapeutic Implications for Antioxidant Treatment-Literature Review.Adv Urol. 2017;2017:4653512. doi: 10.1155/2017/4653512. Epub 2017 Jul 4. Adv Urol. 2017. PMID: 28744308 Free PMC article. Review.
-
Overlapping Science in Radiation and Sulfur Mustard Exposures of Skin and Lung: Consideration of Models, Mechanisms, Organ Systems, and Medical Countermeasures: Overlapping science in radiation and sulfur mustard injuries to lung and skin.Disaster Med Public Health Prep. 2023 Oct 19;17:e552. doi: 10.1017/dmp.2023.176. Disaster Med Public Health Prep. 2023. PMID: 37852927 Free PMC article.
References
-
- Aikawa P, Zhang H, Barbas CS, Pazetti R, Correia C, Mauad T, Silva E, Sannomiya P, Poli-de-Figueiredo LF, Nakagawa NK. The effects of low and high tidal volume and pentoxifylline on intestinal blood flow and leukocyte-endothelial interactions in mechanically ventilated rats. Respir Care. 2011;56:1942–1949. - PubMed
-
- Almario B, Wu S, Peng J, Alapati D, Chen S, Sosenko IR. Pentoxifylline and prevention of hyperoxia-induced lung -injury in neonatal rats. Pediatr Res. 2012;71:583–589. - PubMed
-
- Anderson DR, Taylor SL, Fetterer DP, Holmes WW. Evaluation of protease inhibitors and an antioxidant for treatment of sulfur mustard-induced toxic lung injury. Toxicology. 2009;263:41–46. - PubMed
-
- Barkhordari K, Karimi A, Shafiee A, Soltaninia H, Khatami MR, Abbasi K, Yousefshahi F, Haghighat B, Brown V. Effect of pentoxifylline on preventing acute kidney injury after cardiac surgery by measuring urinary neutrophil gelatinase - associated lipocalin. J Cardiothorac Surg. 2011;6:8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

