Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis
- PMID: 24886976
- PMCID: PMC4075412
- DOI: 10.1186/ar4567
Type 1 regulatory T cells specific for collagen type II as an efficient cell-based therapy in arthritis
Abstract
Introduction: Regulatory T (Treg) cells play a crucial role in preventing autoimmune diseases and are an ideal target for the development of therapies designed to suppress inflammation in an antigen-specific manner. Type 1 regulatory T (Tr1) cells are defined by their capacity to produce high levels of interleukin 10 (IL-10), which contributes to their ability to suppress pathological immune responses in several settings. The aim of this study was to evaluate the therapeutic potential of collagen type II-specific Tr1 (Col-Treg) cells in two models of rheumatoid arthritis (RA) in mice.
Methods: Col-Treg clones were isolated and expanded from collagen-specific TCR transgenic mice. Their cytokine secretion profile and phenotype characterization were studied. The therapeutic potential of Col-Treg cells was evaluated after adoptive transfer in collagen-antibody- and collagen-induced arthritis models. The in vivo suppressive mechanism of Col-Treg clones on effector T-cell proliferation was also investigated.
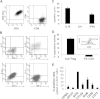
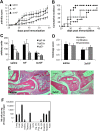
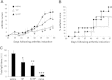
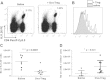
Results: Col-Treg clones are characterized by their specific cytokine profile (IL-10(high)IL-4(neg)IFN-γ(int)) and mediate contact-independent immune suppression. They also share with natural Tregs high expression of GITR, CD39 and granzyme B. A single infusion of Col-Treg cells reduced the incidence and clinical symptoms of arthritis in both preventive and curative settings, with a significant impact on collagen type II antibodies. Importantly, injection of antigen-specific Tr1 cells decreased the proliferation of antigen-specific effector T cells in vivo significantly.
Conclusions: Our results demonstrate the therapeutic potential of Col-Treg cells in two models of RA, providing evidence that Col-Treg could be an efficient cell-based therapy for RA patients whose disease is refractory to current treatments.
Figures

Similar articles
-
Inhibition of Noninfectious Uveitis Using Intravenous Administration of Collagen II-Specific Type 1 Regulatory T Cells.Invest Ophthalmol Vis Sci. 2015 Oct;56(11):6456-66. doi: 10.1167/iovs.15-16883. Invest Ophthalmol Vis Sci. 2015. PMID: 26447979
-
Impact of Schistosoma japonicum infection on collagen-induced arthritis in DBA/1 mice: a murine model of human rheumatoid arthritis.PLoS One. 2011;6(8):e23453. doi: 10.1371/journal.pone.0023453. Epub 2011 Aug 8. PLoS One. 2011. PMID: 21858123 Free PMC article.
-
IL-10 signaling in CD4+ T cells is critical for the pathogenesis of collagen-induced arthritis.Arthritis Res Ther. 2011;13(6):R212. doi: 10.1186/ar3545. Epub 2011 Dec 22. Arthritis Res Ther. 2011. PMID: 22192790 Free PMC article.
-
Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis.Mod Rheumatol. 2009;19(6):581-9. doi: 10.1007/s10165-009-0210-0. Epub 2009 Aug 21. Mod Rheumatol. 2009. PMID: 19697097 Review.
-
Migration and homeostasis of regulatory T cells in rheumatoid arthritis.Front Immunol. 2022 Aug 9;13:947636. doi: 10.3389/fimmu.2022.947636. eCollection 2022. Front Immunol. 2022. PMID: 36016949 Free PMC article. Review.
Cited by
-
Evaluation of the Effects of an Undenatured Collagen Type-2-Based Nutraceutical (ARTHROSHINE® HA²) on Recovery Time after TPLO in Dogs: A Prospective, Randomized Study with Objective Gait Analysis as the Primary Outcome Measure.Animals (Basel). 2024 Jan 18;14(2):298. doi: 10.3390/ani14020298. Animals (Basel). 2024. PMID: 38254467 Free PMC article.
-
Collagen Supplementation for Joint Health: The Link between Composition and Scientific Knowledge.Nutrients. 2023 Mar 8;15(6):1332. doi: 10.3390/nu15061332. Nutrients. 2023. PMID: 36986062 Free PMC article. Review.
-
Extra-thymically induced T regulatory cell subsets: the optimal target for antigen-specific immunotherapy.Immunology. 2015 Jun;145(2):171-81. doi: 10.1111/imm.12458. Immunology. 2015. PMID: 25716063 Free PMC article. Review.
-
Undenatured Type II Collagen (UC-II) in Joint Health and Disease: A Review on the Current Knowledge of Companion Animals.Animals (Basel). 2020 Apr 17;10(4):697. doi: 10.3390/ani10040697. Animals (Basel). 2020. PMID: 32316397 Free PMC article. Review.
-
The effect of oral administration of undenatured type II collagen on monosodium iodoacetate-induced osteoarthritis in young and old rats.Sci Rep. 2023 Apr 20;13(1):6499. doi: 10.1038/s41598-023-33763-2. Sci Rep. 2023. PMID: 37081089 Free PMC article.
References
-
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, Dijkmans BA, Tak PP, Wolbink GJ. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–926. doi: 10.1136/ard.2006.065615. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

