Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer
- PMID: 24887009
- PMCID: PMC4036310
- DOI: 10.1186/1756-9966-33-38
Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer
Abstract
Background: Gastric carcinoma (GC) is a common and lethal malignancy, and epithelial-mesenchymal transition (EMT) is believed to contribute to invasive and metastatic tumor growth. Aquaporin 3 (AQP3) is overexpressed in human GC tissues, while human epidermal growth factor (EGF) and hepatocyte growth factor, which can induce EMT, are able to up-regulate AQP3 expression, subsequently promoting GC cell migration and proliferation. The purpose of this study was to investigate the effects of AQP3 on EMT in human GC.
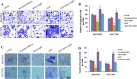
Methods: AQP3 and EMT-related proteins were detected by immunohistochemistry in human GC specimens and their clinical significance evaluated. AQP3 knockdown was attempted using small interfering RNAs, while EGF was used to up-regulate AQP3 expression. Western blotting, real-time quantitative polymerase chain reaction assays and immunofluorescence were used to evaluate changes in expression of AQP3 and EMT-related proteins in the SGC7901 and MGC803 human GC cell lines.
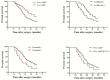
Results: AQP3 up-expression was associated with EMT-related proteins in human GC specimens, which correlated with poor prognosis for GC. AQP3 modulated GC cell proliferation, migration and invasion in vitro, and induced E-cadherin repression. AQP3 also up-regulated the expression of vimentin and fibronectin in vitro. The PI3K/AKT/SNAIL signaling pathway was likely involved in the induction of EMT by AQP3 in GC.
Conclusions: AQP3 promotes EMT in human cases of GC, allowing us to understand the mechanisms of AQP3 in GC progression, thus providing a potential strategy for its treatment.
Figures




References
-
- Yoon CH, Kim MJ, Lee H, Kim RK, Lim EJ, Yoo KC, Lee GH, Cui YH, Oh YS, Gye MC, Lee YY, Park IC, An S, Hwang SG, Park MJ, Suh Y, Lee SJ. PTTG1 promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population. J Biol Chem. 2012;287:19516–19527. doi: 10.1074/jbc.M111.337428. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

