Toxic shock syndrome toxin-1-mediated toxicity inhibited by neutralizing antibodies late in the course of continual in vivo and in vitro exposure
- PMID: 24887085
- PMCID: PMC4073126
- DOI: 10.3390/toxins6061724
Toxic shock syndrome toxin-1-mediated toxicity inhibited by neutralizing antibodies late in the course of continual in vivo and in vitro exposure
Abstract
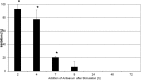
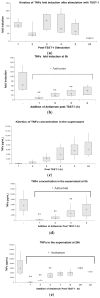
Toxic shock syndrome (TSS) results from the host's overwhelming inflammatory response and cytokine storm mainly due to superantigens (SAgs). There is no effective specific therapy. Application of immunoglobulins has been shown to improve the outcome of the disease and to neutralize SAgs both in vivo and in vitro. However, in most experiments that have been performed, antiserum was either pre-incubated with SAg, or both were applied simultaneously. To mirror more closely the clinical situation, we applied a multiple dose (over five days) lethal challenge in a rabbit model. Treatment with toxic shock syndrome toxin 1 (TSST-1) neutralizing antibody was fully protective, even when administered late in the course of the challenge. Kinetic studies on the effect of superantigen toxins are scarce. We performed in vitro kinetic studies by neutralizing the toxin with antibodies at well-defined time points. T-cell activation was determined by assessing T-cell proliferation (3H-thymidine incorporation), determination of IL-2 release in the cell supernatant (ELISA), and IL-2 gene activation (real-time PCR (RT-PCR)). Here we show that T-cell activation occurs continuously. The application of TSST-1 neutralizing antiserum reduced IL-2 and TNFα release into the cell supernatant, even if added at later time points. Interference with the prolonged stimulation of proinflammatory cytokines is likely to be in vivo relevant, as postexposure treatment protected rabbits against the multiple dose lethal SAg challenge. Our results shed new light on the treatment of TSS by specific antibodies even at late stages of exposure.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

