Reshaping chromatin after DNA damage: the choreography of histone proteins
- PMID: 24887097
- PMCID: PMC5111727
- DOI: 10.1016/j.jmb.2014.05.025
Reshaping chromatin after DNA damage: the choreography of histone proteins
Abstract
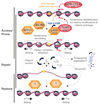
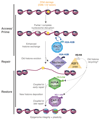
DNA damage signaling and repair machineries operate in a nuclear environment where DNA is wrapped around histone proteins and packaged into chromatin. Understanding how chromatin structure is restored together with the DNA sequence during DNA damage repair has been a topic of intense research. Indeed, chromatin integrity is central to cell functions and identity. However, chromatin shows remarkable plasticity in response to DNA damage. This review presents our current knowledge of chromatin dynamics in the mammalian cell nucleus in response to DNA double strand breaks and UV lesions. I provide an overview of the key players involved in regulating histone dynamics in damaged chromatin regions, focusing on histone chaperones and their concerted action with histone modifiers, chromatin remodelers and repair factors. I also discuss how these dynamics contribute to reshaping chromatin and, by altering the chromatin landscape, may affect the maintenance of epigenetic information.
Keywords: chromatin remodeling; genotoxic stress; histone chaperones; histone modifications; histone variants.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931–54. - PubMed
-
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Nature. 1997;389:251–60. - PubMed
-
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

