Identification of novel SHOX target genes in the developing limb using a transgenic mouse model
- PMID: 24887312
- PMCID: PMC4041798
- DOI: 10.1371/journal.pone.0098543
Identification of novel SHOX target genes in the developing limb using a transgenic mouse model
Abstract
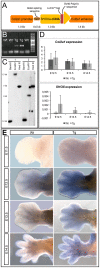
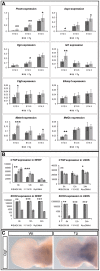
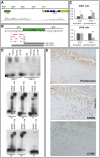
Deficiency of the human short stature homeobox-containing gene (SHOX) has been identified in several disorders characterized by reduced height and skeletal anomalies such as Turner syndrome, Léri-Weill dyschondrosteosis and Langer mesomelic dysplasia as well as isolated short stature. SHOX acts as a transcription factor during limb development and is expressed in chondrocytes of the growth plates. Although highly conserved in vertebrates, rodents lack a SHOX orthologue. This offers the unique opportunity to analyze the effects of human SHOX expression in transgenic mice. We have generated a mouse expressing the human SHOXa cDNA under the control of a murine Col2a1 promoter and enhancer (Tg(Col2a1-SHOX)). SHOX and marker gene expression as well as skeletal phenotypes were characterized in two transgenic lines. No significant skeletal anomalies were found in transgenic compared to wildtype mice. Quantitative and in situ hybridization analyses revealed that Tg(Col2a1-SHOX), however, affected extracellular matrix gene expression during early limb development, suggesting a role for SHOX in growth plate assembly and extracellular matrix composition during long bone development. For instance, we could show that the connective tissue growth factor gene Ctgf, a gene involved in chondrogenic and angiogenic differentiation, is transcriptionally regulated by SHOX in transgenic mice. This finding was confirmed in human NHDF and U2OS cells and chicken micromass culture, demonstrating the value of the SHOX-transgenic mouse for the characterization of SHOX-dependent genes and pathways in early limb development.
Conflict of interest statement
Figures

Similar articles
-
A Track Record on SHOX: From Basic Research to Complex Models and Therapy.Endocr Rev. 2016 Aug;37(4):417-48. doi: 10.1210/er.2016-1036. Epub 2016 Jun 29. Endocr Rev. 2016. PMID: 27355317 Free PMC article. Review.
-
FGFR3 is a target of the homeobox transcription factor SHOX in limb development.Hum Mol Genet. 2011 Apr 15;20(8):1524-35. doi: 10.1093/hmg/ddr030. Epub 2011 Jan 27. Hum Mol Genet. 2011. PMID: 21273290
-
The homeobox transcription factor HOXA9 is a regulator of SHOX in U2OS cells and chicken micromass cultures.PLoS One. 2012;7(9):e45369. doi: 10.1371/journal.pone.0045369. Epub 2012 Sep 20. PLoS One. 2012. PMID: 23028966 Free PMC article.
-
Complete SHOX deficiency causes Langer mesomelic dysplasia.Am J Med Genet. 2002 Jun 15;110(2):158-63. doi: 10.1002/ajmg.10422. Am J Med Genet. 2002. PMID: 12116254
-
[From gene to disease; from SHOX to Lèri-Weill dyschondrosteosis, Turner syndrome and idiopathic short stature].Ned Tijdschr Geneeskd. 2001 Jul 28;145(30):1456-9. Ned Tijdschr Geneeskd. 2001. PMID: 11503314 Review. Dutch.
Cited by
-
SHOX Haploinsufficiency as a Cause of Syndromic and Nonsyndromic Short Stature.Mol Syndromol. 2016 Apr;7(1):3-11. doi: 10.1159/000444596. Epub 2016 Mar 15. Mol Syndromol. 2016. PMID: 27194967 Free PMC article. Review.
-
SHOX haploinsufficiency presenting with isolated short long bones in the second and third trimester.Eur J Hum Genet. 2018 Mar;26(3):350-358. doi: 10.1038/s41431-017-0080-4. Epub 2018 Jan 12. Eur J Hum Genet. 2018. PMID: 29330548 Free PMC article.
-
Identification and Tissue-Specific Characterization of Novel SHOX-Regulated Genes in Zebrafish Highlights SOX Family Members Among Other Genes.Front Genet. 2021 May 27;12:688808. doi: 10.3389/fgene.2021.688808. eCollection 2021. Front Genet. 2021. PMID: 34122528 Free PMC article.
-
Leri-Weill Dyschondrosteosis Caused by a Leaky Homozygous SHOX Splice-Site Variant.Genes (Basel). 2023 Apr 7;14(4):877. doi: 10.3390/genes14040877. Genes (Basel). 2023. PMID: 37107635 Free PMC article.
-
A Track Record on SHOX: From Basic Research to Complex Models and Therapy.Endocr Rev. 2016 Aug;37(4):417-48. doi: 10.1210/er.2016-1036. Epub 2016 Jun 29. Endocr Rev. 2016. PMID: 27355317 Free PMC article. Review.
References
-
- Rao E, Weiss B, Fukami M, Rump A, Niesler B, et al. (1997) Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet 16: 54–63. - PubMed
-
- Shears DJ, Vassal HJ, Goodman FR, Palmer RW, Reardon W, et al. (1998) Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet 19: 70–73. - PubMed
-
- Ross JL, Scott C Jr, Marttila P, Kowal K, Nass A, et al. (2001) Phenotypes Associated with SHOX Deficiency. J Clin Endocrinol Metab 86: 5674–5680. - PubMed
-
- Rappold GA, Ross JL, Blaschke RJ, Blum W (2002) Understanding SHOX deficiency and its role in growth disorders. A reference guide. Oxfordshire, UK: TMG Healthcare Communications Ltd.
-
- Shears DJ, Guillen-Navarro E, Sempere-Miralles M, Domingo-Jimenez R, Scambler PJ, et al. (2002) Pseudodominant inheritance of Langer mesomelic dysplasia caused by a SHOX homeobox missense mutation. Am J Med Genet 110: 153–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

