Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo
- PMID: 24887410
- PMCID: PMC4041793
- DOI: 10.1371/journal.pone.0099029
Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo
Abstract
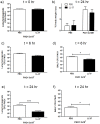
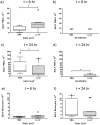
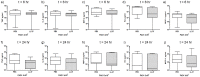
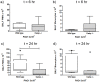
Cathelicidins are multifunctional cationic host-defence peptides (CHDP; also known as antimicrobial peptides) and an important component of innate host defence against infection. In addition to microbicidal potential, these peptides have properties with the capacity to modulate inflammation and immunity. However, the extent to which such properties play a significant role during infection in vivo has remained unclear. A murine model of acute P. aeruginosa lung infection was utilised, demonstrating cathelicidin-mediated enhancement of bacterial clearance in vivo. The delivery of exogenous synthetic human cathelicidin LL-37 was found to enhance a protective pro-inflammatory response to infection, effectively promoting bacterial clearance from the lung in the absence of direct microbicidal activity, with an enhanced early neutrophil response that required both infection and peptide exposure and was independent of native cathelicidin production. Furthermore, although cathelicidin-deficient mice had an intact early cellular inflammatory response, later phase neutrophil response to infection was absent in these animals, with significantly impaired clearance of P. aeruginosa. These findings demonstrate the importance of the modulatory properties of cathelicidins in pulmonary infection in vivo and highlight a key role for cathelicidins in the induction of protective pulmonary neutrophil responses, specific to the infectious milieu. In additional to their physiological roles, CHDP have been proposed as future antimicrobial therapeutics. Elucidating and utilising the modulatory properties of cathelicidins has the potential to inform the development of synthetic peptide analogues and novel therapeutic approaches based on enhancing innate host defence against infection with or without direct microbicidal targeting of pathogens.
Conflict of interest statement
Figures




Similar articles
-
Cathelicidin is a "fire alarm", generating protective NLRP3-dependent airway epithelial cell inflammatory responses during infection with Pseudomonas aeruginosa.PLoS Pathog. 2019 Apr 12;15(4):e1007694. doi: 10.1371/journal.ppat.1007694. eCollection 2019 Apr. PLoS Pathog. 2019. PMID: 30978238 Free PMC article.
-
The human cathelicidin LL-37 preferentially promotes apoptosis of infected airway epithelium.Am J Respir Cell Mol Biol. 2010 Dec;43(6):692-702. doi: 10.1165/rcmb.2009-0250OC. Epub 2010 Jan 22. Am J Respir Cell Mol Biol. 2010. PMID: 20097832 Free PMC article.
-
The Mla pathway is critical for Pseudomonas aeruginosa resistance to outer membrane permeabilization and host innate immune clearance.J Mol Med (Berl). 2017 Oct;95(10):1127-1136. doi: 10.1007/s00109-017-1579-4. Epub 2017 Aug 26. J Mol Med (Berl). 2017. PMID: 28844103 Free PMC article.
-
The human cathelicidin LL-37: a multifunctional peptide involved in infection and inflammation in the lung.Pulm Pharmacol Ther. 2005;18(5):321-7. doi: 10.1016/j.pupt.2005.01.001. Pulm Pharmacol Ther. 2005. PMID: 15939310 Review.
-
The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections.Peptides. 2010 Sep;31(9):1791-8. doi: 10.1016/j.peptides.2010.06.016. Epub 2010 Jun 25. Peptides. 2010. PMID: 20600427 Review.
Cited by
-
On-demand imidazolidinyl urea-based tissue-like, self-healable, and antibacterial hydrogels for infectious wound care.Bioact Mater. 2024 Oct 15;44:116-130. doi: 10.1016/j.bioactmat.2024.10.003. eCollection 2025 Feb. Bioact Mater. 2024. PMID: 39484021 Free PMC article.
-
Cathelicidin-deficient mice exhibit increased survival and upregulation of key inflammatory response genes following cecal ligation and puncture.J Mol Med (Berl). 2017 Sep;95(9):995-1003. doi: 10.1007/s00109-017-1555-z. Epub 2017 Jun 16. J Mol Med (Berl). 2017. PMID: 28623379
-
Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium.Nat Commun. 2016 Feb 29;7:10859. doi: 10.1038/ncomms10859. Nat Commun. 2016. PMID: 26924467 Free PMC article.
-
LL-37 and citrullinated-LL-37 modulate IL-17A/F-mediated responses and selectively suppress Lipocalin-2 in bronchial epithelial cells.J Inflamm (Lond). 2025 May 23;22(1):20. doi: 10.1186/s12950-025-00446-w. J Inflamm (Lond). 2025. PMID: 40410820 Free PMC article.
-
Cathelicidin-mediated lipopolysaccharide signaling via intracellular TLR4 in colonic epithelial cells evokes CXCL8 production.Gut Microbes. 2020 Nov 9;12(1):1785802. doi: 10.1080/19490976.2020.1785802. Epub 2020 Jul 13. Gut Microbes. 2020. PMID: 32658599 Free PMC article.
References
-
- Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415: 389–395. - PubMed
-
- Beaumont PE, Li H, Davidson DJ (2013) LL-37: An Immunomodulatory Antimicrobial Host Defence Peptide. In: Hiemstra PS, Zaat SAJ, Antimicrobial peptides and Innate Immunity. Basel: Springer pp. 97–122.
-
- Bowdish DME, Davidson DJ, Hancock REW (2005) A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci 6: 35–51. - PubMed
-
- Lipsky BA, Holroyd KJ, Zasloff M (2008) Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double-blinded, multicenter trial of pexiganan cream. Clin Infect Dis 47: 1537–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

