Implementing a multifaceted tailored intervention to improve nutrition adequacy in critically ill patients: results of a multicenter feasibility study
- PMID: 24887445
- PMCID: PMC4229943
- DOI: 10.1186/cc13867
Implementing a multifaceted tailored intervention to improve nutrition adequacy in critically ill patients: results of a multicenter feasibility study
Abstract
Introduction: Tailoring interventions to address identified barriers to change may be an effective strategy to implement guidelines and improve practice. However, there is inadequate data to inform the optimal method or level of tailoring. Consequently, we conducted the PERFormance Enhancement of the Canadian nutrition guidelines by a Tailored Implementation Strategy (PERFECTIS) study to determine the feasibility of a multifaceted, interdisciplinary, tailored intervention aimed at improving adherence to critical care nutrition guidelines for the provision of enteral nutrition.
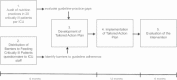
Methods: A before-after study was conducted in seven ICUs from five hospitals in North America. During a 3-month pre-implementation phase, each ICU completed a nutrition practice audit to identify guideline-practice gaps and a barriers assessment to identify obstacles to practice change. During a one day meeting, the results of the audit and barriers assessment were reviewed and used to develop a site-specific tailored action plan. The tailored action plan was then implemented over a 12-month period that included bi-monthly progress meetings. Compliance with the tailored action plan was determined by the proportion of items in the action plan that was completely implemented. We examined acceptability of the intervention through staff responses to an evaluation questionnaire. In addition, the nutrition practice audit and barriers survey were repeated at the end of the implementation phase to determine changes in barriers and nutrition practices.
Results: All five sites successfully completed all aspects of the study. However, their ability to fully implement all of their developed action plans varied from 14% to 75% compliance. Nurses, on average, rated the study-related activities and resources as 'somewhat useful' and a third of respondents 'agreed' or 'strongly agreed' that their nutrition practice had changed as a result of the intervention. We observed a statistically significant 10% (Site range -4.3% to -26.0%) decrease in overall barriers score, and a non-significant 6% (Site range -1.5% to 17.9%) and 4% (-8.3% to 18.2%) increase in the adequacy of total nutrition from calories and protein, respectively.
Conclusions: The multifaceted tailored intervention appears to be feasible but further refinement is warranted prior to testing the effectiveness of the approach on a larger scale.
Trial registration: ClinicalTrials.gov NCT01168128. Registered 21 July 2010.
Figures


Similar articles
-
Improving the provision of enteral nutrition in the intensive care unit: a description of a multifaceted intervention tailored to overcome local barriers.Nutr Clin Pract. 2014 Feb;29(1):110-7. doi: 10.1177/0884533613516512. Epub 2013 Dec 16. Nutr Clin Pract. 2014. PMID: 24344255
-
Current status and influencing factors of barriers to enteral feeding of critically ill patients: A multicenter study.J Clin Nurs. 2019 Feb;28(3-4):677-685. doi: 10.1111/jocn.14667. Epub 2018 Sep 23. J Clin Nurs. 2019. PMID: 30182514
-
The validation of a questionnaire to assess barriers to enteral feeding in critically ill patients: a multicenter international survey.BMC Health Serv Res. 2014 May 1;14:197. doi: 10.1186/1472-6963-14-197. BMC Health Serv Res. 2014. PMID: 24885039 Free PMC article.
-
Bridging the guideline-practice gap in critical care nutrition: a review of guideline implementation studies.JPEN J Parenter Enteral Nutr. 2010 Nov-Dec;34(6):653-9. doi: 10.1177/0148607110361907. JPEN J Parenter Enteral Nutr. 2010. PMID: 21097765 Review.
-
Seven Deadly Sins of Nutrition Therapy in Critically Ill Patients.Nutr Clin Pract. 2020 Apr;35(2):205-210. doi: 10.1002/ncp.10430. Epub 2019 Oct 22. Nutr Clin Pract. 2020. PMID: 31642115 Review.
Cited by
-
Trends in guideline implementation: an updated scoping review.Implement Sci. 2022 Jul 23;17(1):50. doi: 10.1186/s13012-022-01223-6. Implement Sci. 2022. PMID: 35870974 Free PMC article.
-
Making sense of the outcome of a rehabilitation implementation trial in the intensive care unit: Mixed methods.South Afr J Crit Care. 2025 May 19;41(1):e2695. doi: 10.7196/SAJCC.2695.v41i1.2695. eCollection 2025. South Afr J Crit Care. 2025. PMID: 40874037 Free PMC article.
-
Evaluation of a minimal sedation protocol using ICU sedative consumption as a monitoring tool: a quality improvement multicenter project.Crit Care. 2014 Oct 24;18(5):580. doi: 10.1186/s13054-014-0580-3. Crit Care. 2014. PMID: 25673553 Free PMC article.
-
Extrinsic and intrinsic factors acting as barriers or facilitators in nurses' implementation of clinical practice guidelines: a mixed-method systematic review.Acta Biomed. 2022 Jul 1;93(3):e2022252. doi: 10.23750/abm.v93i3.12942. Acta Biomed. 2022. PMID: 35775756 Free PMC article.
-
Evidence on nutritional therapy practice guidelines and implementation in adult critically ill patients: A systematic scoping review.Curationis. 2019 Dec 13;42(1):e1-e13. doi: 10.4102/curationis.v42i1.1973. Curationis. 2019. PMID: 31833375 Free PMC article.
References
-
- Critical Illness Evidence-based Nutrition Practice Guideline 2006 (Update 2012) [ http://andevidencelibrary.com/topic.cfm?cat=4840]
-
- Doig G, Simpson F. Evidence-based guidelines for nutritional support of the critically ill: results of a bi-national guideline development conference. 2005. [ http://www.evidencebased.net/files/]
-
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–373. - PubMed
-
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Ebner C, Hartl W, Heymann C, Spies C. ESPEN. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–223. - PubMed
-
- McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

