Stilbene induced inhibition of androgen receptor dimerization: implications for AR and ARΔLBD-signalling in human prostate cancer cells
- PMID: 24887556
- PMCID: PMC4041728
- DOI: 10.1371/journal.pone.0098566
Stilbene induced inhibition of androgen receptor dimerization: implications for AR and ARΔLBD-signalling in human prostate cancer cells
Abstract
Background: Advanced castration resistant prostate cancer (CRPC) is often characterized by an increase of C-terminally truncated, constitutively active androgen receptor (AR) variants. Due to the absence of a ligand binding domain located in the AR-C-terminus, these receptor variants (also termed ARΔLBD) are unable to respond to all classical forms of endocrine treatments like surgical/chemical castration and/or application of anti-androgens.
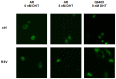
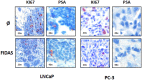
Methodology: In this study we tested the effects of the naturally occurring stilbene resveratrol (RSV) and (E)-4-(2, 6-Difluorostyryl)-N, N-dimethylaniline, a fluorinated dialkylaminostilbene (FIDAS) on AR- and ARΔLBD in prostate cancer cells. The ability of the compounds to modulate transcriptional activity of AR and the ARΔLBD-variant Q640X was shown by reporter gene assays. Expression of endogenous AR and ARΔLBD mRNA and protein levels were determined by qRT-PCR and Western Blot. Nuclear translocation of AR-molecules was analyzed by fluorescence microscopy. AR and ARΔLBD/Q640X homo-/heterodimer formation was assessed by mammalian two hybrid assays. Biological activity of both compounds in vivo was demonstrated using a chick chorioallantoic membrane xenograft assay.
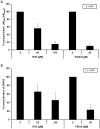
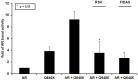
Results: The stilbenes RSV and FIDAS were able to significantly diminish AR and Q640X-signalling. Successful inhibition of the Q640X suggests that RSV and FIDAS are not interfering with the AR-ligand binding domain like all currently available anti-hormonal drugs. Repression of AR and Q640X-signalling by RSV and FIDAS in prostate cancer cells was caused by an inhibition of the AR and/or Q640X-dimerization. Although systemic bioavailability of both stilbenes is very low, both compounds were also able to downregulate tumor growth and AR-signalling in vivo.
Conclusion: RSV and FIDAS are able to inhibit the dimerization of AR and ARΔLBD molecules suggesting that stilbenes might serve as lead compounds for a novel generation of AR-inhibitors.
Conflict of interest statement
Figures


Similar articles
-
AR-Q640X, a model to study the effects of constitutively active C-terminally truncated AR variants in prostate cancer cells.World J Urol. 2012 Jun;30(3):333-9. doi: 10.1007/s00345-012-0842-0. Epub 2012 Feb 24. World J Urol. 2012. PMID: 22362413
-
Effects of sorafenib on C-terminally truncated androgen receptor variants in human prostate cancer cells.Int J Mol Sci. 2012;13(9):11530-11542. doi: 10.3390/ijms130911530. Epub 2012 Sep 14. Int J Mol Sci. 2012. PMID: 23109869 Free PMC article.
-
Inhibition of IGF-1R diminishes transcriptional activity of the androgen receptor and its constitutively active, C-terminally truncated counterparts Q640X and AR-V7.World J Urol. 2016 May;34(5):633-9. doi: 10.1007/s00345-015-1674-5. Epub 2015 Aug 29. World J Urol. 2016. PMID: 26318637
-
Experimental evidence of persistent androgen-receptor-dependency in castration-resistant prostate cancer.Int J Mol Sci. 2013 Jul 26;14(8):15615-35. doi: 10.3390/ijms140815615. Int J Mol Sci. 2013. PMID: 23896594 Free PMC article. Review.
-
C-terminally truncated constitutively active androgen receptor variants and their biologic and clinical significance in castration-resistant prostate cancer.J Steroid Biochem Mol Biol. 2017 Feb;166:38-44. doi: 10.1016/j.jsbmb.2016.06.008. Epub 2016 Jun 21. J Steroid Biochem Mol Biol. 2017. PMID: 27345700 Review.
Cited by
-
Androgen receptor aberrations in the era of abiraterone and enzalutamide.World J Urol. 2016 Mar;34(3):297-303. doi: 10.1007/s00345-015-1624-2. Epub 2015 Jun 23. World J Urol. 2016. PMID: 26100946 Review.
-
Overview on the complexity of androgen receptor-targeted therapy for prostate cancer.Cancer Cell Int. 2015 Feb 4;15:7. doi: 10.1186/s12935-014-0153-1. eCollection 2015. Cancer Cell Int. 2015. PMID: 25705125 Free PMC article.
-
Gnetin C in Cancer and Other Diseases: What Do We Know So Far?Nutrients. 2025 Feb 28;17(5):863. doi: 10.3390/nu17050863. Nutrients. 2025. PMID: 40077738 Free PMC article. Review.
-
The Role of Resveratrol in Human Male Fertility.Molecules. 2021 Apr 24;26(9):2495. doi: 10.3390/molecules26092495. Molecules. 2021. PMID: 33923359 Free PMC article. Review.
-
Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study.J Clin Oncol. 2019 Jun 10;37(17):1499-1511. doi: 10.1200/JCO.18.02462. Epub 2019 Apr 26. J Clin Oncol. 2019. PMID: 31026211 Free PMC article.
References
-
- Feldman BJ, Feldman D (2001) The development of androgen-Independent prostate cancer. Nat Rev Cancer 1: 34–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

