A non-canonical NRPS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum
- PMID: 24887561
- PMCID: PMC4041764
- DOI: 10.1371/journal.pone.0098212
A non-canonical NRPS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum
Abstract
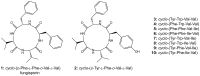
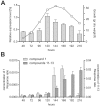
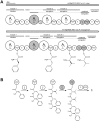
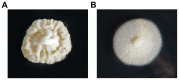
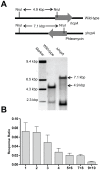
The filamentous fungus Penicillium chrysogenum harbors an astonishing variety of nonribosomal peptide synthetase genes, which encode proteins known to produce complex bioactive metabolites from simple building blocks. Here we report a novel non-canonical tetra-modular nonribosomal peptide synthetase (NRPS) with microheterogenicity of all involved adenylation domains towards their respective substrates. By deleting the putative gene in combination with comparative metabolite profiling various unique cyclic and derived linear tetrapeptides were identified which were associated with this NRPS, including fungisporin. In combination with substrate predictions for each module, we propose a mechanism for a 'trans-acting' adenylation domain.
Conflict of interest statement
Figures
References
-
- Schwarzer D, Finking R, Marahiel MA (2003) Nonribosomal peptides: from genes to products. Nat Prod Rep 20: 275–287. - PubMed
-
- Mootz HD, Schwarzer D, Marahiel MA (2002) Ways of assembling complex natural products on modular nonribosomal peptide synthetases. Chembiochem 3: 490–504. - PubMed
-
- Fischbach MA, Walsh CT (2006) Assembly-line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem Rev 106: 3468–3496. - PubMed
-
- Shaw-Reid CA, Kelleher NL, Losey HC, Gehring AM, Berg C, et al. (1999) Assembly line enzymology by multimodular nonribosomal peptide synthetases: the thioesterase domain of E. coli EntF catalyzes both elongation and cyclolactonization. Chem Biol 6: 385–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

