Intracellular pH in sperm physiology
- PMID: 24887564
- PMCID: PMC4146485
- DOI: 10.1016/j.bbrc.2014.05.100
Intracellular pH in sperm physiology
Abstract
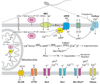
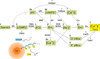
Intracellular pH (pHi) regulation is essential for cell function. Notably, several unique sperm ion transporters and enzymes whose elimination causes infertility are either pHi dependent or somehow related to pHi regulation. Amongst them are: CatSper, a Ca(2+) channel; Slo3, a K(+) channel; the sperm-specific Na(+)/H(+) exchanger and the soluble adenylyl cyclase. It is thus clear that pHi regulation is of the utmost importance for sperm physiology. This review briefly summarizes the key components involved in pHi regulation, their characteristics and participation in fundamental sperm functions such as motility, maturation and the acrosome reaction.
Keywords: Intracellular pH; Sperm; Sperm chemotaxis; Sperm motility.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

