Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study
- PMID: 24887569
- PMCID: PMC4075416
- DOI: 10.1186/cc13874
Does contemporary vancomycin dosing achieve therapeutic targets in a heterogeneous clinical cohort of critically ill patients? Data from the multinational DALI study
Abstract
Introduction: The objective of this study was to describe the pharmacokinetics of vancomycin in ICU patients and to examine whether contemporary antibiotic dosing results in concentrations that have been associated with favourable response.
Methods: The Defining Antibiotic Levels in Intensive Care (DALI) study was a prospective, multicentre pharmacokinetic point-prevalence study. Antibiotic dosing was as per the treating clinician either by intermittent bolus or continuous infusion. Target trough concentration was defined as ≥15 mg/L and target pharmacodynamic index was defined as an area under the concentration-time curve over a 24-hour period divided by the minimum inhibitory concentration of the suspected bacteria (AUC0-24/MIC ratio) >400 (assuming MIC ≤1 mg/L).
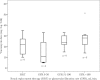
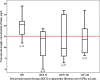
Results: Data of 42 patients from 26 ICUs were eligible for analysis. A total of 24 patients received vancomycin by continuous infusion (57%). Daily dosage of vancomycin was 27 mg/kg (interquartile range (IQR) 18 to 32), and not different between patients receiving intermittent or continuous infusion. Trough concentrations were highly variable (median 27, IQR 8 to 23 mg/L). Target trough concentrations were achieved in 57% of patients, but more frequently in patients receiving continuous infusion (71% versus 39%; P = 0.038). Also the target AUC0-24/MIC ratio was reached more frequently in patients receiving continuous infusion (88% versus 50%; P = 0.008). Multivariable logistic regression analysis with adjustment by the propensity score could not confirm continuous infusion as an independent predictor of an AUC0-24/MIC >400 (odds ratio (OR) 1.65, 95% confidence interval (CI) 0.2 to 12.0) or a Cmin ≥15 mg/L (OR 1.8, 95% CI 0.4 to 8.5).
Conclusions: This study demonstrated large interindividual variability in vancomycin pharmacokinetic and pharmacodynamic target attainment in ICU patients. These data suggests that a re-evaluation of current vancomycin dosing recommendations in critically ill patients is needed to more rapidly and consistently achieve sufficient vancomycin exposure.
Figures



References
-
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. - DOI - PubMed
-
- Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–327. doi: 10.1086/600877. - DOI - PubMed
-
- Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O'Sullivan MV, Anderson TL, Roberts SA, Warren SJ, Gao W, Howden BP, Johnson PD. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57:1654–1663. doi: 10.1128/AAC.01485-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

