Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis
- PMID: 24887616
- PMCID: PMC4109030
- DOI: 10.7326/M13-2263
Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis
Abstract
Background: The U.S. Preventive Services Task Force recommends against routine screening for colorectal cancer (CRC) in adequately screened persons older than 75 years but does not address the appropriateness of screening in elderly persons without previous screening.
Objective: To determine at what ages CRC screening should be considered in unscreened elderly persons and to determine which test is indicated at each age.
Design: Microsimulation modeling study.
Data sources: Observational and experimental studies.
Target population: Unscreened persons aged 76 to 90 years with no, moderate, and severe comorbid conditions.
Time horizon: Lifetime.
Perspective: Societal.
Intervention: One-time colonoscopy, sigmoidoscopy, or fecal immunochemical test (FIT) screening.
Outcome measures: Quality-adjusted life-years gained, costs, and costs per quality-adjusted life-year gained.
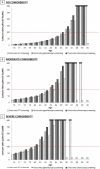
Results of base-case analysis: In unscreened elderly persons with no comorbid conditions, CRC screening was cost-effective up to age 86 years. Screening with colonoscopy was indicated up to age 83 years, sigmoidoscopy was indicated at age 84 years, and FIT was indicated at ages 85 and 86 years. In unscreened persons with moderate comorbid conditions, screening was cost-effective up to age 83 years (colonoscopy indicated up to age 80 years, sigmoidoscopy at age 81 years, and FIT at ages 82 and 83 years). In unscreened persons with severe comorbid conditions, screening was cost-effective up to age 80 years (colonoscopy indicated up to age 77 years, sigmoidoscopy at age 78 years, and FIT at ages 79 and 80 years).
Results of sensitivity analyses: Results were most sensitive to assuming a lower willingness to pay per quality-adjusted life-year gained.
Limitation: Only persons at average risk for CRC were considered.
Conclusion: In unscreened elderly persons CRC screening should be considered well beyond age 75 years. A colonoscopy is indicated at most ages.
Primary funding source: National Cancer Institute.
Figures

Comment in
-
A "green banana" worth buying in older age: colorectal cancer screening for persons older than 75 years without previous screening.Ann Intern Med. 2014 Jun 3;160(11):804-5. doi: 10.7326/M14-0869. Ann Intern Med. 2014. PMID: 24887619 No abstract available.
-
"Old classic cars" are hidden treasures: colorectal cancer screening should be considered in unscreened persons over age 75.Turk J Gastroenterol. 2014 Jun;25(3):349-50. doi: 10.5152/tjg.2014.0015. Turk J Gastroenterol. 2014. PMID: 25141335 No abstract available.
References
-
- Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–37. - PubMed
-
- Rozen P, Liphshitz I, Barchana M. The changing epidemiology of colorectal cancer and its relevance for adapting screening guidelines and methods. Eur J Cancer Prev. 2011;20(1):46–53. - PubMed
-
- Shellnut JK, Wasvary HJ, Grodsky MB, Boura JA, Priest SG. Evaluating the age distribution of patients with colorectal cancer: are the United States Preventative Services Task Force guidelines for colorectal cancer screening appropriate? Dis Colon Rectum. 2010;53(1):5–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
