Under a nonadherent state, bone marrow mesenchymal stem cells can be efficiently induced into functional islet-like cell clusters to normalize hyperglycemia in mice: a control study
- PMID: 24887638
- PMCID: PMC4076641
- DOI: 10.1186/scrt455
Under a nonadherent state, bone marrow mesenchymal stem cells can be efficiently induced into functional islet-like cell clusters to normalize hyperglycemia in mice: a control study
Abstract
Introduction: Bone marrow mesenchymal stem cells (BMSCs) possess low immunogenicity and immunosuppression as an allograft, can differentiate into insulin-producing cells (IPCs) by in vitro induction, and may be a valuable cell source to regenerate pancreatic islets. However, the very low differentiation efficiency of BMSCs towards IPCs under adherent induction has thus far hindered the clinical exploitation of these cells. The aim of this study is to explore a new way to efficiently induce BMSCs into IPCs and lay the groundwork for their clinical exploitation.
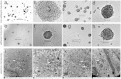
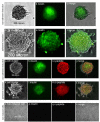
Methods: In comparison with adherent induction, BMSCs of human first-trimester abortus (hfBMSCs) under a nonadherent state were induced towards IPCs in noncoated plastic dishes using a three-stage induction procedure developed by the authors. Induction effects were evaluated by statistics of the cell clustering rate of induced cells, and ultrastructural observation, dithizone staining, quantitative polymerase chain reaction and immunofluorescence assay, insulin and c-peptide release under glucose stimulus of cell clusters, as well as transplantation test of the cell clusters in diabetic model mice.
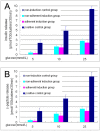
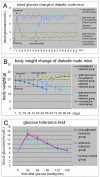
Results: With (6.175 ± 0.263) × 105 cells in 508.5 ± 24.5 cell clusters, (3.303 ± 0.331) × 105 single cells and (9.478 ± 0.208) × 105 total cell count on average, 65.08 ± 2.98% hfBMSCs differentiated into pancreatic islet-like cell clusters after nonadherent induction. With (3.993 ± 0.344) × 105 cells in 332.3 ± 41.6 cell clusters, (5.437 ± 0.434) × 105 single cells and (9.430 ± 0.340) × 105 total cell count on average, 42.37 ± 3.70% hfBMSCs differentiated into pancreatic islet-like cell clusters after adherent induction (P < 0.01, n = 10). The former is significantly higher than the latter. Calculated according to the cell clustering rate and IPC percentage in the cell clusters, 29.80 ± 3.95% hfBMSCs differentiated into IPCs after nonadherent induction and 18.40 ± 2.08% hfBMSCs differentiated into IPCs after adherent induction (P < 0.01, n = 10), the former significantly higher than the latter. The cell clusters expressed a broad gene profile related to pancreatic islet cells, released insulin and c-peptide in a glucose concentration-dependent manner, and normalized hyperglycemia of streptozocin-induced mice for at least 80 days following xenograft. Blood glucose of grafted mice rose again after their graft removed. A series of examination of the grafts showed that transplanted cells produced human insulin in recipients.
Conclusions: Our studies demonstrate that nonadherent induction can greatly promote BMSCs to form pancreatic islet-like cell clusters, thereby improving the differentiation efficiency of BMSCs towards IPCs.
Figures




Similar articles
-
Pancreatic islet-like clusters from bone marrow mesenchymal stem cells of human first-trimester abortus can cure streptozocin-induced mouse diabetes.Rejuvenation Res. 2010 Dec;13(6):695-706. doi: 10.1089/rej.2009.1016. Epub 2011 Jan 4. Rejuvenation Res. 2010. PMID: 21204652
-
Insulin-Producing Cells Differentiated from Human Bone Marrow Mesenchymal Stem Cells In Vitro Ameliorate Streptozotocin-Induced Diabetic Hyperglycemia.PLoS One. 2016 Jan 12;11(1):e0145838. doi: 10.1371/journal.pone.0145838. eCollection 2016. PLoS One. 2016. PMID: 26756576 Free PMC article.
-
Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice.Cell Transplant. 2013;22(1):133-45. doi: 10.3727/096368912X647162. Epub 2012 Jun 15. Cell Transplant. 2013. PMID: 22710060 Free PMC article.
-
From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Progress and Challenges.Stem Cell Rev Rep. 2020 Dec;16(6):1156-1172. doi: 10.1007/s12015-020-10036-3. Stem Cell Rev Rep. 2020. PMID: 32880857 Free PMC article. Review.
-
Differentiation of Mesenchymal Stem Cells from Humans and Animals into Insulin-producing Cells: An Overview In Vitro Induction Forms.Curr Stem Cell Res Ther. 2021;16(6):695-709. doi: 10.2174/1574888X16666201229124429. Curr Stem Cell Res Ther. 2021. PMID: 33372881 Review.
Cited by
-
Stem cells to replace or regenerate the diabetic pancreas: Huge potential & existing hurdles.Indian J Med Res. 2016 Mar;143(3):267-74. doi: 10.4103/0971-5916.182615. Indian J Med Res. 2016. PMID: 27241638 Free PMC article. Review.
-
Transcriptome analysis of the transdifferentiation of canine BMSCs into insulin producing cells.BMC Genomics. 2021 Feb 25;22(1):134. doi: 10.1186/s12864-021-07426-3. BMC Genomics. 2021. PMID: 33632121 Free PMC article.
-
Efficiency of Stem Cell (SC) Differentiation into Insulin-Producing Cells for Treating Diabetes: a Systematic Review.Stem Cells Int. 2021 Feb 25;2021:6652915. doi: 10.1155/2021/6652915. eCollection 2021. Stem Cells Int. 2021. PMID: 33727934 Free PMC article. Review.
-
In vitro differentiation of human umbilical cord Wharton's jelly mesenchymal stromal cells to insulin producing clusters.World J Clin Cases. 2015 Jul 16;3(7):640-9. doi: 10.12998/wjcc.v3.i7.640. World J Clin Cases. 2015. PMID: 26244156 Free PMC article.
-
Mesenchymal Stem Cells: An Excellent Candidate for the Treatment of Diabetes Mellitus.Int J Endocrinol. 2021 May 28;2021:9938658. doi: 10.1155/2021/9938658. eCollection 2021. Int J Endocrinol. 2021. PMID: 34135959 Free PMC article. Review.
References
-
- Aanstoot HJ, Anderson BJ, Daneman D, Danne T, Donaghue K, Kaufman F, Rea RR, Uchigata Y. The global burden of youth diabetes: perspectives and potential. Pediatr Diabetes. 2007;8:1–44. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

