The purinergic neurotransmitter revisited: a single substance or multiple players?
- PMID: 24887688
- PMCID: PMC4185222
- DOI: 10.1016/j.pharmthera.2014.05.012
The purinergic neurotransmitter revisited: a single substance or multiple players?
Abstract
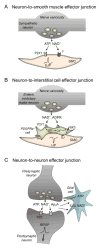
The past half century has witnessed tremendous advances in our understanding of extracellular purinergic signaling pathways. Purinergic neurotransmission, in particular, has emerged as a key contributor in the efficient control mechanisms in the nervous system. The identity of the purine neurotransmitter, however, remains controversial. Identifying it is difficult because purines are present in all cell types, have a large variety of cell sources, and are released via numerous pathways. Moreover, studies on purinergic neurotransmission have relied heavily on indirect measurements of integrated postjunctional responses that do not provide direct information for neurotransmitter identity. This paper discusses experimental support for adenosine 5'-triphosphate (ATP) as a neurotransmitter and recent evidence for possible contribution of other purines, in addition to or instead of ATP, in chemical neurotransmission in the peripheral, enteric and central nervous systems. Sites of release and action of purines in model systems such as vas deferens, blood vessels, urinary bladder and chromaffin cells are discussed. This is preceded by a brief discussion of studies demonstrating storage of purines in synaptic vesicles. We examine recent evidence for cell type targets (e.g., smooth muscle cells, interstitial cells, neurons and glia) for purine neurotransmitters in different systems. This is followed by brief discussion of mechanisms of terminating the action of purine neurotransmitters, including extracellular nucleotide hydrolysis and possible salvage and reuptake in the cell. The significance of direct neurotransmitter release measurements is highlighted. Possibilities for involvement of multiple purines (e.g., ATP, ADP, NAD(+), ADP-ribose, adenosine, and diadenosine polyphosphates) in neurotransmission are considered throughout.
Keywords: ADP-ribose; ATP; Adenosine; NAD; Nervous system; Purinergic neurotransmission.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



Similar articles
-
Adenosine 5-diphosphate-ribose is a neural regulator in primate and murine large intestine along with β-NAD(+).J Physiol. 2012 Apr 15;590(8):1921-41. doi: 10.1113/jphysiol.2011.222414. Epub 2012 Feb 20. J Physiol. 2012. PMID: 22351627 Free PMC article.
-
Neuronal and extraneuronal release of ATP and NAD(+) in smooth muscle.IUBMB Life. 2012 Oct;64(10):817-24. doi: 10.1002/iub.1076. Epub 2012 Sep 3. IUBMB Life. 2012. PMID: 22941916 Free PMC article. Review.
-
Loss of nitric oxide-mediated inhibition of purine neurotransmitter release in the colon in the absence of interstitial cells of Cajal.Am J Physiol Gastrointest Liver Physiol. 2017 Nov 1;313(5):G419-G433. doi: 10.1152/ajpgi.00045.2017. Epub 2017 Jul 13. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 28705804 Free PMC article.
-
Purinergic transmission in blood vessels.Auton Neurosci. 2015 Sep;191:48-66. doi: 10.1016/j.autneu.2015.04.007. Epub 2015 Apr 25. Auton Neurosci. 2015. PMID: 26004513 Review.
-
Introduction to Purinergic Signaling.Methods Mol Biol. 2020;2041:1-15. doi: 10.1007/978-1-4939-9717-6_1. Methods Mol Biol. 2020. PMID: 31646477
Cited by
-
A commonly used ecto-ATPase inhibitor, ARL-67156, blocks degradation of ADP more than the degradation of ATP in murine colon.Neurogastroenterol Motil. 2016 Sep;28(9):1370-81. doi: 10.1111/nmo.12836. Epub 2016 Apr 5. Neurogastroenterol Motil. 2016. PMID: 27060478 Free PMC article.
-
LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia.Mol Brain. 2016 Apr 23;9:44. doi: 10.1186/s13041-016-0226-2. Mol Brain. 2016. PMID: 27107575 Free PMC article.
-
Activity-induced Ca2+ signaling in perisynaptic Schwann cells of the early postnatal mouse is mediated by P2Y1 receptors and regulates muscle fatigue.Elife. 2018 Jan 31;7:e30839. doi: 10.7554/eLife.30839. Elife. 2018. PMID: 29384476 Free PMC article.
-
Sensory Neurons, PIEZO Channels and PAC1 Receptors Regulate the Mechanosensitive Release of Soluble Ectonucleotidases in the Murine Urinary Bladder Lamina Propria.Int J Mol Sci. 2023 Apr 15;24(8):7322. doi: 10.3390/ijms24087322. Int J Mol Sci. 2023. PMID: 37108490 Free PMC article.
-
Ventricular Arrhythmias and Myocardial Infarction: Electrophysiological and Neuroimmune Mechanisms.Biomedicines. 2025 May 23;13(6):1290. doi: 10.3390/biomedicines13061290. Biomedicines. 2025. PMID: 40564008 Free PMC article. Review.
References
-
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. - PubMed
-
- Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

