Mammary stem cells and the differentiation hierarchy: current status and perspectives
- PMID: 24888586
- PMCID: PMC4052761
- DOI: 10.1101/gad.242511.114
Mammary stem cells and the differentiation hierarchy: current status and perspectives
Abstract
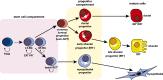
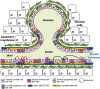
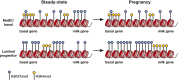
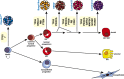
The mammary epithelium is highly responsive to local and systemic signals, which orchestrate morphogenesis of the ductal tree during puberty and pregnancy. Based on transplantation and lineage tracing studies, a hierarchy of stem and progenitor cells has been shown to exist among the mammary epithelium. Lineage tracing has highlighted the existence of bipotent mammary stem cells (MaSCs) in situ as well as long-lived unipotent cells that drive morphogenesis and homeostasis of the ductal tree. Moreover, there is accumulating evidence for a heterogeneous MaSC compartment comprising fetal MaSCs, slow-cycling cells, and both long-term and short-term repopulating cells. In parallel, diverse luminal progenitor subtypes have been identified in mouse and human mammary tissue. Elucidation of the normal cellular hierarchy is an important step toward understanding the "cells of origin" and molecular perturbations that drive breast cancer.
Keywords: breast cancer; development; epigenome; lineage tracing; mammary stem cell; steroid hormone.
© 2014 Visvader and Stingl; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Asselin-Labat ML, Shackleton M, Stingl J, Vaillant F, Forrest NC, Eaves CJ, Visvader JE, Lindeman GJ 2006. Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 98: 1011–1014 - PubMed
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. 2007. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9: 201–209 - PubMed
-
- Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, et al. 2010. Control of mammary stem cell function by steroid hormone signalling. Nature 465: 798–802 - PubMed
-
- Asselin-Labat ML, Sutherland KD, Vaillant F, Gyorki DE, Wu D, Holroyd S, Breslin K, Ward T, Shi W, Bath ML, et al. 2011. Gata-3 negatively regulates the tumor-initiating capacity of mammary luminal progenitor cells and targets the putative tumor suppressor caspase-14. Mol Cell Biol 31: 4609–4622 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
