Mechanotransduction in the muscle spindle
- PMID: 24888691
- PMCID: PMC4281366
- DOI: 10.1007/s00424-014-1536-9
Mechanotransduction in the muscle spindle
Abstract
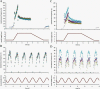
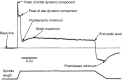
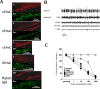
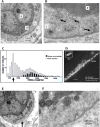
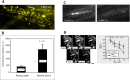
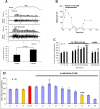
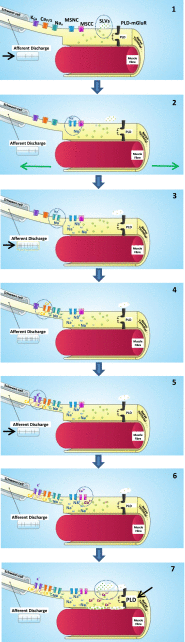
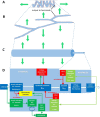
The focus of this review is on the principal sensory ending of the mammalian muscle spindle, known as the primary ending. The process of mechanosensory transduction in the primary ending is examined under five headings: (i) action potential responses to defined mechanical stimuli-representing the ending's input-output properties; (ii) the receptor potential-including the currents giving rise to it; (iii) sensory-terminal deformation-measurable changes in the shape of the primary-ending terminals correlated with intrafusal sarcomere length, and what may cause them; (iv) putative stretch-sensitive channels-pharmacological and immunocytochemical clues to their identity; and (v) synaptic-like vesicles-the physiology and pharmacology of an intrinsic glutamatergic system in the primary and other mechanosensory endings, with some thoughts on the possible role of the system. Thus, the review highlights spindle stretch-evoked output is the product of multi-ionic receptor currents plus complex and sophisticated regulatory gain controls, both positive and negative in nature, as befits its status as the most complex sensory organ after the special senses.
Figures


References
-
- Akoev GN, Alekseev NP, Krylov BV. Mechanoreceptors: their functional organisation. Berlin: Springer-Verlag; 1988.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

