Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer
- PMID: 24888810
- PMCID: PMC4067944
- DOI: 10.1200/JCO.2013.54.4932
Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer
Abstract
Purpose: Figitumumab (CP-751,871), a fully human immunoglobulin G2 monoclonal antibody, inhibits the insulin-like growth factor 1 receptor (IGF-1R). Our multicenter, randomized, phase III study compared figitumumab plus chemotherapy with chemotherapy alone as first-line treatment in patients with advanced non-small-cell lung cancer (NSCLC).
Patients and methods: Patients with stage IIIB/IV or recurrent NSCLC disease with nonadenocarcinoma histology received open-label figitumumab (20 mg/kg) plus paclitaxel (200 mg/m(2)) and carboplatin (area under the concentration-time curve, 6 mg · min/mL) or paclitaxel and carboplatin alone once every 3 weeks for up to six cycles. The primary end point was overall survival (OS).
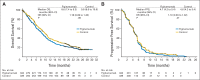
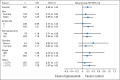
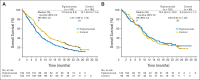
Results: Of 681 randomly assigned patients, 671 received treatment. The study was closed early by an independent Data Safety Monitoring Committee because of futility and an increased incidence of serious adverse events (SAEs) and treatment-related deaths with figitumumab. Median OS was 8.6 months for figitumumab plus chemotherapy and 9.8 months for chemotherapy alone (hazard ratio [HR], 1.18; 95% CI, 0.99 to 1.40; P = .06); median progression-free survival was 4.7 months (95% CI, 4.2 to 5.4) and 4.6 months (95% CI, 4.2 to 5.4), respectively (HR, 1.10; P = .27); the objective response rates were 33% and 35%, respectively. The respective rates of all-causality SAEs were 66% and 51%; P < .01). Treatment-related grade 5 adverse events were also more common with figitumumab (5% v 1%; P < .01).
Conclusion: Adding figitumumab to standard chemotherapy failed to increase OS in patients with advanced nonadenocarcinoma NSCLC. Further clinical development of figitumumab is not being pursued.
Trial registration: ClinicalTrials.gov NCT00596830.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- National Cancer Institute. Bethesda, MD: National Cancer Institute; [accessed October 20, 2011]. PDQ Non-Small Cell Lung Cancer Treatment. http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell-lung/hea....
-
- Pollak M. Targeting insulin and insulin-like growth factor signalling in oncology. Curr Opin Pharmacol. 2008;8:384–392. - PubMed
-
- Favoni RE, de Cupis A, Ravera F, et al. Expression and function of the insulin-like growth factor I system in human non-small-cell lung cancer and normal lung cell lines. Int J Cancer. 1994;56:858–866. - PubMed
-
- Kaiser U, Schardt C, Brandscheidt D, et al. Expression of insulin-like growth factor receptors I and II in normal human lung and in lung cancer. J Cancer Res Clin Oncol. 1993;119:665–668. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

