Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas
- PMID: 24888813
- PMCID: PMC4067943
- DOI: 10.1200/JCO.2013.54.0526
Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas
Abstract
Purpose: Diffuse brainstem gliomas (BSGs) and other high-grade gliomas (HGGs) of childhood carry a dismal prognosis despite current treatments, and new therapies are needed. Having identified a series of glioma-associated antigens (GAAs) commonly overexpressed in pediatric gliomas, we initiated a pilot study of subcutaneous vaccinations with GAA epitope peptides in HLA-A2-positive children with newly diagnosed BSG and HGG.
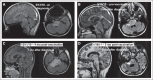
Patients and methods: GAAs were EphA2, interleukin-13 receptor alpha 2 (IL-13Rα2), and survivin, and their peptide epitopes were emulsified in Montanide-ISA-51 and given every 3 weeks with intramuscular polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose for eight courses, followed by booster vaccinations every 6 weeks. Primary end points were safety and T-cell responses against vaccine-targeted GAA epitopes. Treatment response was evaluated clinically and by magnetic resonance imaging.
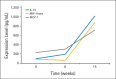
Results: Twenty-six children were enrolled, 14 with newly diagnosed BSG treated with irradiation and 12 with newly diagnosed BSG or HGG treated with irradiation and concurrent chemotherapy. No dose-limiting non-CNS toxicity was encountered. Five children had symptomatic pseudoprogression, which responded to dexamethasone and was associated with prolonged survival. Only two patients had progressive disease during the first two vaccine courses; 19 had stable disease, two had partial responses, one had a minor response, and two had prolonged disease-free status after surgery. Enzyme-linked immunosorbent spot analysis in 21 children showed positive anti-GAA immune responses in 13: to IL-13Rα2 in 10, EphA2 in 11, and survivin in three.
Conclusion: GAA peptide vaccination in children with gliomas is generally well tolerated and has preliminary evidence of immunologic and clinical responses. Careful monitoring and management of pseudoprogression is essential.
Trial registration: ClinicalTrials.gov NCT01130077.
© 2014 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



Similar articles
-
Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas.Neuro Oncol. 2016 Aug;18(8):1157-68. doi: 10.1093/neuonc/now026. Epub 2016 Mar 15. Neuro Oncol. 2016. PMID: 26984745 Free PMC article.
-
Antigen-specific immunoreactivity and clinical outcome following vaccination with glioma-associated antigen peptides in children with recurrent high-grade gliomas: results of a pilot study.J Neurooncol. 2016 Dec;130(3):517-527. doi: 10.1007/s11060-016-2245-3. Epub 2016 Sep 13. J Neurooncol. 2016. PMID: 27624914 Free PMC article.
-
Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma.J Clin Oncol. 2011 Jan 20;29(3):330-6. doi: 10.1200/JCO.2010.30.7744. Epub 2010 Dec 13. J Clin Oncol. 2011. PMID: 21149657 Free PMC article. Clinical Trial.
-
Brain tumor immunotherapy with type-1 polarizing strategies.Ann N Y Acad Sci. 2009 Sep;1174:18-23. doi: 10.1111/j.1749-6632.2009.04932.x. Ann N Y Acad Sci. 2009. PMID: 19769732 Review.
-
Challenges in the development of a survivin vaccine (SurVaxM) for malignant glioma.Expert Rev Vaccines. 2014 Mar;13(3):377-85. doi: 10.1586/14760584.2014.881255. Expert Rev Vaccines. 2014. PMID: 24521310 Review.
Cited by
-
Targeting the innate immune system as immunotherapy for acute myeloid leukemia.Front Oncol. 2015 Apr 9;5:83. doi: 10.3389/fonc.2015.00083. eCollection 2015. Front Oncol. 2015. PMID: 25914882 Free PMC article. Review.
-
Making a Cold Tumor Hot: The Role of Vaccines in the Treatment of Glioblastoma.Front Oncol. 2021 May 10;11:672508. doi: 10.3389/fonc.2021.672508. eCollection 2021. Front Oncol. 2021. PMID: 34041034 Free PMC article. Review.
-
Eph Receptor Tyrosine Kinases in Tumor Immunity.Cancer Res. 2016 Nov 15;76(22):6452-6457. doi: 10.1158/0008-5472.CAN-16-1521. Epub 2016 Nov 3. Cancer Res. 2016. PMID: 27811149 Free PMC article. Review.
-
Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy.J Hematol Oncol. 2019 Nov 29;12(1):128. doi: 10.1186/s13045-019-0813-7. J Hematol Oncol. 2019. PMID: 31783889 Free PMC article. Review.
-
Dendritic Cell-Based and Other Vaccination Strategies for Pediatric Cancer.Cancers (Basel). 2019 Sep 19;11(9):1396. doi: 10.3390/cancers11091396. Cancers (Basel). 2019. PMID: 31546858 Free PMC article. Review.
References
-
- Pollack IF. Brain tumors in children. N Engl J Med. 1994;331:1500–1507. - PubMed
-
- Jennings MT, Freeman ML, Murray MJ. Strategies in the treatment of diffuse pontine gliomas: The therapeutic role of hyperfractionated radiotherapy and chemotherapy. J Neurooncol. 1996;28:207–222. - PubMed
-
- Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: A Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

