Auto-induction of phase I and phase II metabolism of artemisinin in healthy Chinese subjects after oral administration of a new artemisinin-piperaquine fixed combination
- PMID: 24889062
- PMCID: PMC4055232
- DOI: 10.1186/1475-2875-13-214
Auto-induction of phase I and phase II metabolism of artemisinin in healthy Chinese subjects after oral administration of a new artemisinin-piperaquine fixed combination
Abstract
Background: Artequick is a relatively inexpensive artemisinin (Qing-hao-su; QHS)-based combination therapy (ACT) that contains QHS and piperaquine (PQ), which has not been widely used because of the decreased concentration level of QHS after repeated oral administrations for five to seven days as a monotherapy. This study was designed to evaluate the potential auto-induction metabolism of QHS in healthy Chinese adults after a two-day oral administration of QHS-PQ. The effect of QHS-PQ on the activity of the CYP2B6 and CYP3A4 was also investigated.
Methods: Fourteen healthy Chinese subjects received two-day oral doses of QHS-PQ (Artequick). A two-drug cocktail consisting of bupropion and midazolam was used to assess the activities of CYP2B6 and CYP3A, respectively. Plasma samples were analysed for QHS and its phase I/II metabolites, probe drugs and their metabolites, using a validated liquid chromatography tandem mass spectrometric (LC-MS) method.
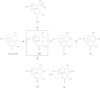
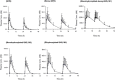
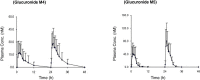
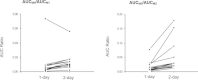
Results: Four major phase I metabolites of QHS (M1-M3 and deoxy-QHS) and two subsequent phase II metabolites (M4-M5) were detected in human plasma after oral administrations of QHS-PQ. The AUC0-t of the QHS and its phase I metabolites decreased significantly (P < 0.05) with increased oral clearance (CL/F) after two-day oral doses of QHS-PQ, whereas its phase II metabolites exhibited higher AUC (P < 0.01). The phase I metabolic capability, calculated by the AUC0-t ratio of all phase I metabolites to QHS, increased 1.5-fold after the repeated dose (P < 0.01), and the phase II metabolic capability increased 1.5-fold for M4 and 3.0-fold for M5. The enzyme activity of CYP2B6 and CYP3A4 increased 2.1-fold and 3.2-fold, respectively, after two-day oral doses of QHS-PQ.
Conclusions: The auto-induction of both phase I and phase II metabolism of QHS was present in healthy Chinese subjects after a recommended two-day oral dose of QHS-PQ. The auto-induction metabolism also existed for phase I metabolites of QHS. The enzyme activity of CYP2B6 and CYP3A4 was induced after the two-day oral doses of QHS-PQ. Based on these results, the alternative common three-day regimen for QHS-PQ could probably lead to lower bioavailability of QHS and higher potential of drug-drug interaction caused by the induction of drug-metabolizing enzymes.
Figures


References
-
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Fleck M, Bajad S, Dang G, Dengrove D, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. - DOI - PubMed
-
- Salman S, Page-Sharp M, Batty KT, Kose K, Griffin S, Siba PM, Ilett KF, Mueller I, Davis TM. Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother. 2012;56:3288–3297. doi: 10.1128/AAC.06232-11. - DOI - PMC - PubMed
-
- Krudsood S, Tangpukdee N, Thanchatwet V, Wilairatana P, Srivilairit S, Pothipak N, Jianping S, Guoqiao L, Brittenham GM, Looareesuwan S. Dose ranging studies of new artemisinin-piperaquine fixed combinations compared to standard regimens of artemisisnin combination therapies for acute uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 2007;38:971–978. - PMC - PubMed
-
- Zhu DY, Huang BS, Chen ZL, Yin ML, Yang YM, Dai ML, Wang BD, Huang ZH. Isolation and identification of the metabolite of artemisinin in human (in Chinese) Zhongguo Yao Li Xue Bao. 1983;4:194–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

