Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes
- PMID: 24889220
- PMCID: PMC4179455
- DOI: 10.1016/j.virol.2014.03.002
Endocytosis-mediated HIV-1 entry and its significance in the elusive behavior of the virus in astrocytes
Abstract
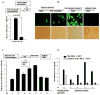
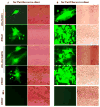
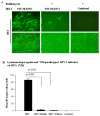
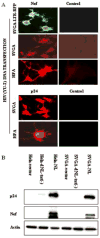
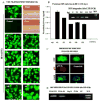
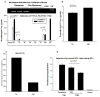
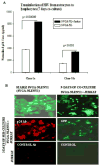
Astrocytes protect neurons but also evoke a proinflammatory response to injury and viral infections including HIV. We investigated the mechanism of HIV-1 infection in primary astrocytes, which showed minimal but productive viral infection independent of CXCR4. As with ectopic-CD4-expressing astrocytes, lysosomotropic agents led to increased HIV-1 infection in wild-type but not Rabs 5, 7, and 11-ablated astrocytes. Instead, HIV-1 infection was decreased in Rab-depleted astrocytes, corroborating viral entry by endocytosis. HIV-1 produced persistent infection in astrocytes (160 days); no evidence of latent infection was seen. Notably, one caveat is that endosomal modifiers enhanced wild-type HIV-1 infection (M- and T-tropic) in astrocytes, suggesting endocytic entry of the virus. Impeding endocytosis by inhibition of Rab 5, 7 or 11 will inhibit HIV infection in astrocytes. Although the contribution of such low-level infection in astrocytes to neurological complications is unclear, it may serve as an elusive viral reservoir in the central nervous system.
Keywords: HIV-1 persistence; HIV-LTR; LSP1; Lysosomotropic agents; Proteasome-inhibitors; Rab; TNPO3.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Productive HIV infection in astrocytes can be established via a nonclassical mechanism.AIDS. 2020 Jun 1;34(7):963-978. doi: 10.1097/QAD.0000000000002512. AIDS. 2020. PMID: 32379159 Free PMC article.
-
Endocytosis of human immunodeficiency virus 1 (HIV-1) in astrocytes: a fiery path to its destination.Microb Pathog. 2015 Jan;78:1-6. doi: 10.1016/j.micpath.2014.11.003. Epub 2014 Nov 4. Microb Pathog. 2015. PMID: 25448132 Free PMC article. Review.
-
CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor.J Virol. 2004 Apr;78(8):4120-33. doi: 10.1128/jvi.78.8.4120-4133.2004. J Virol. 2004. PMID: 15047828 Free PMC article.
-
HIV-1 endocytosis in astrocytes: a kiss of death or survival of the fittest?Neurosci Res. 2014 Nov;88:16-22. doi: 10.1016/j.neures.2014.08.013. Epub 2014 Sep 8. Neurosci Res. 2014. PMID: 25219546 Free PMC article. Review.
-
Emergence of CD4 independence envelopes and astrocyte infection in R5 simian-human immunodeficiency virus model of encephalitis.J Virol. 2014 Aug;88(15):8407-20. doi: 10.1128/JVI.01237-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829360 Free PMC article.
Cited by
-
Blood-brain barrier pericytes as a target for HIV-1 infection.Brain. 2019 Mar 1;142(3):502-511. doi: 10.1093/brain/awy339. Brain. 2019. PMID: 30668645 Free PMC article.
-
Next-Generation Human Cerebral Organoids as Powerful Tools To Advance NeuroHIV Research.mBio. 2021 Aug 31;12(4):e0068021. doi: 10.1128/mBio.00680-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253056 Free PMC article. Review.
-
Astrocytes, HIV and the Glymphatic System: A Disease of Disrupted Waste Management?Front Cell Infect Microbiol. 2020 Sep 29;10:523379. doi: 10.3389/fcimb.2020.523379. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33134185 Free PMC article. Review.
-
Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4.AIDS. 2015 Apr 24;29(7):755-66. doi: 10.1097/QAD.0000000000000605. AIDS. 2015. PMID: 25985398 Free PMC article.
-
Productive HIV infection in astrocytes can be established via a nonclassical mechanism.AIDS. 2020 Jun 1;34(7):963-978. doi: 10.1097/QAD.0000000000002512. AIDS. 2020. PMID: 32379159 Free PMC article.
References
-
- Bencheikh M, Bentsman G, Sarkissian N, Canki M, Volsky DJ. Replication of different clones of human immunodeficiency virus type 1 in primary fetal human astrocytes: enhancement of viral gene expression by Nef. J Neurovirol. 1999;5:115–124. - PubMed
-
- Bounou S, Giguere JF, Cantin R, Gilbert C, Imbeault M, Martin G, Tremblay MJ. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. Faseb J. 2004;18:1294–1296. - PubMed
-
- Boutet A, Salim H, Taoufik Y, Lledo PM, Vincent JD, Delfraissy JF, Tardieu M. Isolated human astrocytes are not susceptible to infection by M- and T-tropic HIV-1 strains despite functional expression of the chemokine receptors CCR5 and CXCR4. Glia. 2001;34:165–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

