The crucial role of bile acids in the entry of porcine enteric calicivirus
- PMID: 24889246
- PMCID: PMC4064365
- DOI: 10.1016/j.virol.2014.04.002
The crucial role of bile acids in the entry of porcine enteric calicivirus
Abstract
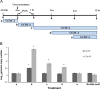
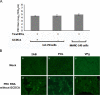
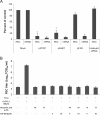
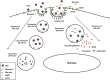
Replication of porcine enteric calicivirus (PEC) in LLC-PK cells is dependent on the presence of bile acids in the medium. However, the mechanism of bile acid-dependent PEC replication is unknown. Understanding of bile acid-mediated PEC replication may provide insight into cultivating related human noroviruses, currently uncultivable, which are the major cause of viral gastroenteritis outbreaks in humans. Our results demonstrated that while uptake of PEC into the endosomes does not require bile acids, the presence of bile acids is critical for viral escape from the endosomes into cell cytoplasm to initiate viral replication. We also demonstrated that bile acid transporters including the sodium-taurocholate co-transporting polypeptide and the apical sodium-dependent bile acid transporter are important in exerting the effects of bile acids in PEC replication in cells. In summary, our results suggest that bile acids play a critical role in virus entry for successful replication.
Keywords: Bile acids; Bile transporters; Endosomal escape; Porcine enteric calicivirus; Virus entry.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Ceramide formation mediated by acid sphingomyelinase facilitates endosomal escape of caliciviruses.Virology. 2015 Sep;483:218-28. doi: 10.1016/j.virol.2015.04.022. Epub 2015 May 15. Virology. 2015. PMID: 25985440 Free PMC article.
-
Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1.Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8733-8. doi: 10.1073/pnas.0401126101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161971 Free PMC article.
-
Phosphatidylinositol 3-Kinase/Akt and MEK/ERK Signaling Pathways Facilitate Sapovirus Trafficking and Late Endosomal Acidification for Viral Uncoating in LLC-PK Cells.J Virol. 2018 Nov 27;92(24):e01674-18. doi: 10.1128/JVI.01674-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282712 Free PMC article.
-
Bile Goes Viral.Viruses. 2021 May 27;13(6):998. doi: 10.3390/v13060998. Viruses. 2021. PMID: 34071855 Free PMC article. Review.
-
Bile acid transporters: structure, function, regulation and pathophysiological implications.Pharm Res. 2007 Oct;24(10):1803-23. doi: 10.1007/s11095-007-9289-1. Epub 2007 Apr 3. Pharm Res. 2007. PMID: 17404808 Review.
Cited by
-
Bile acids promote the caveolae-associated entry of swine acute diarrhea syndrome coronavirus in porcine intestinal enteroids.PLoS Pathog. 2022 Jun 13;18(6):e1010620. doi: 10.1371/journal.ppat.1010620. eCollection 2022 Jun. PLoS Pathog. 2022. PMID: 35696443 Free PMC article.
-
In Vitro Replication of Human Norovirus.Viruses. 2019 Jun 12;11(6):547. doi: 10.3390/v11060547. Viruses. 2019. PMID: 31212759 Free PMC article. Review.
-
Early Porcine Sapovirus Infection Disrupts Tight Junctions and Uses Occludin as a Coreceptor.J Virol. 2019 Feb 5;93(4):e01773-18. doi: 10.1128/JVI.01773-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463963 Free PMC article.
-
Calicivirus RNA-Dependent RNA Polymerases: Evolution, Structure, Protein Dynamics, and Function.Front Microbiol. 2019 Jun 6;10:1280. doi: 10.3389/fmicb.2019.01280. eCollection 2019. Front Microbiol. 2019. PMID: 31244803 Free PMC article. Review.
-
Initial Evaluation of Antibody-conjugates Modified with Viral-derived Peptides for Increasing Cellular Accumulation and Improving Tumor Targeting.J Vis Exp. 2018 Mar 8;(133):55440. doi: 10.3791/55440. J Vis Exp. 2018. PMID: 29578523 Free PMC article.
References
-
- Alpini G., Glaser S., Baiocchi L., Francis H., Xia X., Lesage G. Secretin activation of the apical Na+-dependent bile acid transporter is associated with cholehepatic shunting in rats. Hepatology. 2005;41:1037–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

