Synthesis of the first radiolabeled 188Re N-heterocyclic carbene complex and initial studies on its potential use in radiopharmaceutical applications
- PMID: 24889257
- PMCID: PMC4381871
- DOI: 10.1002/jlcr.3203
Synthesis of the first radiolabeled 188Re N-heterocyclic carbene complex and initial studies on its potential use in radiopharmaceutical applications
Abstract
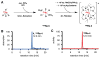
A novel approach towards the synthesis of radiolabeled organometallic rhenium complexes is presented. We successfully synthesized and analyzed the first (188)Re-labeled N-heterocyclic biscarbene complex, trans-dioxobis(1,1'-methylene-bis(3,3'-diisopropylimidazolium-2-ylidene))(188)rhenium(V) hexafluorophosphate ((188)Re-4) via transmetalation using an air-stable and moisture-stable silver(I) biscarbene complex. In order to assess the viability of this complex as a potential lead structure for in vivo applications, the stability of the (188)Re-NHC complex was tested in physiologically relevant media. Ultimately, our studies illustrate that the complex we synthesized dissociates rapidly and is therefore unsuitable for use in radiopharmaceuticals. However, it is clear that the transmetalation approach we have developed is a rapid, robust, and mild method for the synthesis of new (188)Re-labeled carbene complexes.
Keywords: 188Re carbene complex; N-heterocyclic carbene; radiopharmaceutical application; transmetalation.
Copyright © 2014 John Wiley & Sons, Ltd.
Conflict of interest statement
Figures




References
-
- Hom RK, Katzenellenbogen JA. Nucl Med Biol. 1997;24:485–498. - PubMed
-
- Jürgens S, Herrmann WA, Kühn FE. J Organomet Chem. 2014;751:83–89.
-
- Dilworth JR, Parrott SJ. Chem Soc Rev. 1998;27:43–55.
- Volkert WA, Hoffman TJ. Chem Rev. 1999;99:2269–2292. - PubMed
-
- Deutsch E, Libson KF, Vanderheyden JL, Ketring AR, Maxon HR. Nucl Med Biol. 1986;13:465–477.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

