Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia
- PMID: 24889329
- PMCID: PMC4190903
- DOI: 10.1111/jcmm.12304
Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia
Abstract
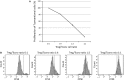
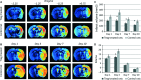
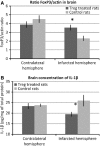
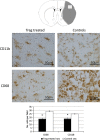
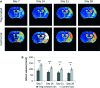
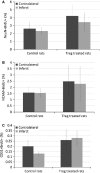
Brain ischaemia (stroke) triggers an intense inflammatory response predominately mediated by the accumulation of inflammatory cells and mediators in the ischaemic brain. In this context, regulatory T (Treg) cells, a subpopulation of CD4(+) T cells with immunosuppressive and anti-inflammatory properties, are activated in the late stages of the disease. To date, the potential therapeutic usefulness of Treg cells has not been tested. In this study, we aimed to investigate whether Treg cells exert protection/repair following stroke. Both the adoptive transfer of Treg cells into ischaemic rats and the stimulation of endogenous T-cell proliferation using a CD28 superagonist reduced the infarct size at 3-28 days following the ischaemic insult. Moreover, T cell-treated animals had higher levels of FoxP3 and lower levels of IL-1β, CD11b+ and CD68+ cells in the infarcted hemisphere when compared with control animals. However, T-cell treatment did not alter the rate of proliferation of NeuN-, NCAM- or CD31-positive cells, thereby ruling out neurogenesis and angiogenesis in protection. These results suggest that adoptive transfer of T cells is a promising therapeutic strategy against the neurological consequences of stroke.
Keywords: cerebrovascular disease/stroke; immunomodulation; inflammation; regulatory T cells.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. - PubMed
-
- Brea D, Sobrino T, Ramos-Cabrer P, et al. Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc Dis. 2009;27:48–64. - PubMed
-
- Yan J, Greer JM, Etherington K, et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol. 2009;206:112–7. - PubMed
-
- Emsley HC, Smith CJ, Gavin CM, et al. An early and sustained peripheral inflammatory response in acute ischaemic stroke: relationships with infection and atherosclerosis. J Neuroimmunol. 2003;139:93–101. - PubMed
-
- Rodríguez-Yáñez M, Castillo J. Role of inflammatory markers in brain ischemia. Curr Opin Neurol. 2008;21:353–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

