Photoinduced transformations of stiff-stilbene-based discrete metallacycles to metallosupramolecular polymers
- PMID: 24889610
- PMCID: PMC4066485
- DOI: 10.1073/pnas.1408620111
Photoinduced transformations of stiff-stilbene-based discrete metallacycles to metallosupramolecular polymers
Abstract
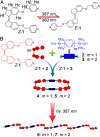
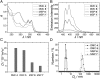
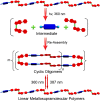
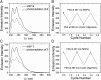
Control over structural transformations in supramolecular entities by external stimuli is critical for the development of adaptable and functional soft materials. Herein, we have designed and synthesized a dipyridyl donor containing a central Z-configured stiff-stilbene unit that self-assembles in the presence of two 180° di-Pt(II) acceptors to produce size-controllable discrete organoplatinum(II) metallacycles with high efficiency by means of the directional-bonding approach. These discrete metallacycles undergo transformation into extended metallosupramolecular polymers upon the conformational switching of the dipyridyl ligand from Z-configured (0°) to E-configured (180°) when photoirradiated. This transformation is accompanied by interesting morphological changes at nanoscopic length scales. The discrete metallacycles aggregate to spherical nanoparticles that evolve into long nanofibers upon polymer formation. These fibers can be reversibly converted to cyclic oligomers by changing the wavelength of irradiation, which reintroduces Z-configured building blocks owing to the reversible nature of stiff-stilbene photoisomerization. The design strategy defined here represents a novel self-assembly pathway to deliver advanced supramolecular assemblies by means of photocontrol.
Keywords: dynamic materials; metal coordination; photoirradiation; reversibility; supramolecular coordination complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Construction of Smart Supramolecular Polymeric Hydrogels Cross-linked by Discrete Organoplatinum(II) Metallacycles via Post-Assembly Polymerization.J Am Chem Soc. 2016 Apr 13;138(14):4927-37. doi: 10.1021/jacs.6b01089. Epub 2016 Mar 29. J Am Chem Soc. 2016. PMID: 27011050
-
Supramolecular transformations within discrete coordination-driven supramolecular architectures.Chem Soc Rev. 2016 May 3;45(9):2656-93. doi: 10.1039/c5cs00301f. Chem Soc Rev. 2016. PMID: 27009833 Review.
-
From Photoinduced Supramolecular Polymerization to Responsive Organogels.J Am Chem Soc. 2021 Apr 21;143(15):5990-5997. doi: 10.1021/jacs.1c01802. Epub 2021 Apr 8. J Am Chem Soc. 2021. PMID: 33830767 Free PMC article.
-
Metallosupramolecular Architectures Obtained from Poly-N-heterocyclic Carbene Ligands.Acc Chem Res. 2017 Sep 19;50(9):2167-2184. doi: 10.1021/acs.accounts.7b00158. Epub 2017 Aug 25. Acc Chem Res. 2017. PMID: 28841284
-
Stiff-Stilbene Photoswitches: From Fundamental Studies to Emergent Applications.Angew Chem Int Ed Engl. 2020 Aug 3;59(32):13192-13202. doi: 10.1002/anie.202001031. Epub 2020 Jun 2. Angew Chem Int Ed Engl. 2020. PMID: 32222016 Free PMC article. Review.
Cited by
-
Synthesis of Metallopolymers and Direct Visualization of the Single Polymer Chain.J Am Chem Soc. 2020 Apr 1;142(13):6196-6205. doi: 10.1021/jacs.0c00110. Epub 2020 Mar 19. J Am Chem Soc. 2020. PMID: 32150680 Free PMC article.
-
Thermally-induced atropisomerism promotes metal-organic cage construction.Nat Commun. 2023 Dec 9;14(1):8166. doi: 10.1038/s41467-023-43756-4. Nat Commun. 2023. PMID: 38071355 Free PMC article.
-
Photocontrol of Anion Binding Affinity to a Bis-urea Receptor Derived from Stiff-Stilbene.Org Lett. 2017 Jan 20;19(2):324-327. doi: 10.1021/acs.orglett.6b03423. Epub 2017 Jan 11. Org Lett. 2017. PMID: 28074657 Free PMC article.
-
All-visible-light-driven stiff-stilbene photoswitches.Chem Sci. 2024 Mar 27;15(18):6763-6769. doi: 10.1039/d4sc00983e. eCollection 2024 May 8. Chem Sci. 2024. PMID: 38725493 Free PMC article.
-
Phototriggered Supramolecular Assembly.ACS Omega. 2020 Dec 8;5(50):32140-32148. doi: 10.1021/acsomega.0c04919. eCollection 2020 Dec 22. ACS Omega. 2020. PMID: 33376852 Free PMC article. Review.
References
-
- Dublin SN, Conticello VP. Design of a selective metal ion switch for self-assembly of peptide-based fibrils. J Am Chem Soc. 2008;130(1):49–51. - PubMed
-
- Kudernac T, et al. Electrically driven directional motion of a four-wheeled molecule on a metal surface. Nature. 2011;479(7372):208–211. - PubMed
-
- Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chem Soc Rev. 2012;41(18):6042–6065. - PubMed
-
- Göstl R, Senf A, Hecht S. Remote-controlling chemical reactions by light: Towards chemistry with high spatio-temporal resolution. Chem Soc Rev. 2014;43(6):1982–1996. - PubMed
-
- Hosono N, et al. Large-area three-dimensional molecular ordering of a polymer brush by one-step processing. Science. 2010;330(6005):808–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

