Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program
- PMID: 24889617
- PMCID: PMC4066505
- DOI: 10.1073/pnas.1405723111
Contact inhibition and high cell density deactivate the mammalian target of rapamycin pathway, thus suppressing the senescence program
Abstract
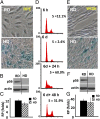
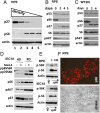
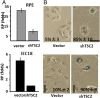
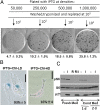
During cell cycle arrest caused by contact inhibition (CI), cells do not undergo senescence, thus resuming proliferation after replating. The mechanism of senescence avoidance during CI is unknown. Recently, it was demonstrated that the senescence program, namely conversion from cell cycle arrest to senescence (i.e., geroconversion), requires mammalian target of rapamycin (mTOR). Geroconversion can be suppressed by serum starvation, rapamycin, and hypoxia, which all inhibit mTOR. Here we demonstrate that CI, as evidenced by p27 induction in normal cells, was associated with inhibition of the mTOR pathway. Furthermore, CI antagonized senescence caused by CDK inhibitors. Stimulation of mTOR in contact-inhibited cells favored senescence. In cancer cells lacking p27 induction and CI, mTOR was still inhibited in confluent culture as a result of conditioning of the medium. This inhibition of mTOR suppressed p21-induced senescence. Also, trapping of malignant cells among contact-inhibited normal cells antagonized p21-induced senescence. Thus, we identified two nonmutually exclusive mechanisms of mTOR inhibition in high cell density: (i) CI associated with p27 induction in normal cells and (ii) conditioning of the medium, especially in cancer cells. Both mechanisms can coincide in various proportions in various cells. Our work explains why CI is reversible and, most importantly, why cells avoid senescence in vivo, given that cells are contact-inhibited in the organism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. - PubMed
-
- Deng Q, Liao R, Wu BL, Sun P. High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J Biol Chem. 2004;279(2):1050–1059. - PubMed
-
- Demidenko ZN, Blagosklonny MV. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle. 2008;7(21):3355–3361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

