Pathway for Mn-cluster oxidation by tyrosine-Z in the S2 state of photosystem II
- PMID: 24889635
- PMCID: PMC4066536
- DOI: 10.1073/pnas.1401719111
Pathway for Mn-cluster oxidation by tyrosine-Z in the S2 state of photosystem II
Abstract
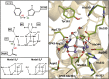
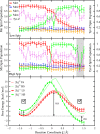
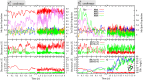
Water oxidation in photosynthetic organisms occurs through the five intermediate steps S0-S4 of the Kok cycle in the oxygen evolving complex of photosystem II (PSII). Along the catalytic cycle, four electrons are subsequently removed from the Mn4CaO5 core by the nearby tyrosine Tyr-Z, which is in turn oxidized by the chlorophyll special pair P680, the photo-induced primary donor in PSII. Recently, two Mn4CaO5 conformations, consistent with the S2 state (namely, S2(A) and S2(B) models) were suggested to exist, perhaps playing a different role within the S2-to-S3 transition. Here we report multiscale ab initio density functional theory plus U simulations revealing that upon such oxidation the relative thermodynamic stability of the two previously proposed geometries is reversed, the S2(B) state becoming the leading conformation. In this latter state a proton coupled electron transfer is spontaneously observed at ∼100 fs at room temperature dynamics. Upon oxidation, the Mn cluster, which is tightly electronically coupled along dynamics to the Tyr-Z tyrosyl group, releases a proton from the nearby W1 water molecule to the close Asp-61 on the femtosecond timescale, thus undergoing a conformational transition increasing the available space for the subsequent coordination of an additional water molecule. The results can help to rationalize previous spectroscopic experiments and confirm, for the first time to our knowledge, that the water-splitting reaction has to proceed through the S2(B) conformation, providing the basis for a structural model of the S3 state.
Keywords: QM/MM; molecular dynamics; photosynthesis; reaction mechanisms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
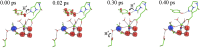

Similar articles
-
Characterization of the Sr(2+)- and Cd(2+)-Substituted Oxygen-Evolving Complex of Photosystem II by Quantum Mechanics/Molecular Mechanics Calculations.Biochemistry. 2015 Sep 29;54(38):5959-68. doi: 10.1021/acs.biochem.5b00797. Epub 2015 Sep 18. Biochemistry. 2015. PMID: 26346422
-
Sequential and Coupled Proton and Electron Transfer Events in the S2 → S3 Transition of Photosynthetic Water Oxidation Revealed by Time-Resolved X-ray Absorption Spectroscopy.Biochemistry. 2016 Dec 20;55(50):6996-7004. doi: 10.1021/acs.biochem.6b01078. Epub 2016 Dec 5. Biochemistry. 2016. PMID: 27992997
-
The collapse of the tyrosine Z*-Mn spin-spin interaction above approximately 100 K reveals the spectrum of tyrosine Z*. An application of rapid-scan EPR to the study of intermediates of the water splitting mechanism of photosystem II.Biochemistry. 2007 Dec 18;46(50):14335-41. doi: 10.1021/bi7018767. Epub 2007 Nov 17. Biochemistry. 2007. PMID: 18020377
-
The O2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data.Acc Chem Res. 2017 Jan 17;50(1):41-48. doi: 10.1021/acs.accounts.6b00405. Epub 2016 Dec 21. Acc Chem Res. 2017. PMID: 28001034 Review.
-
Eight steps preceding O-O bond formation in oxygenic photosynthesis--a basic reaction cycle of the Photosystem II manganese complex.Biochim Biophys Acta. 2007 Jun;1767(6):472-83. doi: 10.1016/j.bbabio.2007.02.022. Epub 2007 Mar 12. Biochim Biophys Acta. 2007. PMID: 17442260 Review.
Cited by
-
Five-coordinate MnIV intermediate in the activation of nature's water splitting cofactor.Proc Natl Acad Sci U S A. 2019 Aug 20;116(34):16841-16846. doi: 10.1073/pnas.1817526116. Epub 2019 Aug 7. Proc Natl Acad Sci U S A. 2019. PMID: 31391299 Free PMC article.
-
Photosystem II: Probing Protons and Breaking Barriers.Biochemistry. 2025 May 6;64(9):1895-1906. doi: 10.1021/acs.biochem.5c00112. Epub 2025 Apr 7. Biochemistry. 2025. PMID: 40193597 Review.
-
Artificial photosynthesis: understanding water splitting in nature.Interface Focus. 2015 Jun 6;5(3):20150009. doi: 10.1098/rsfs.2015.0009. Interface Focus. 2015. PMID: 26052426 Free PMC article. Review.
-
Reversible Structural Isomerization of Nature's Water Oxidation Catalyst Prior to O-O Bond Formation.J Am Chem Soc. 2022 Jul 6;144(26):11736-11747. doi: 10.1021/jacs.2c03528. Epub 2022 Jun 24. J Am Chem Soc. 2022. PMID: 35748306 Free PMC article.
-
Structural isomers of the S2 state in photosystem II: do they exist at room temperature and are they important for function?Physiol Plant. 2019 May;166(1):60-72. doi: 10.1111/ppl.12947. Epub 2019 Mar 15. Physiol Plant. 2019. PMID: 30793319 Free PMC article.
References
-
- Barber J. Photosynthetic energy conversion: Natural and artificial. Chem Soc Rev. 2009;38(1):185–196. - PubMed
-
- Kanan MW, Nocera DG. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ Science. 2008;321(5892):1072–1075. - PubMed
-
- Kanan MW, Surendranath Y, Nocera DG. Cobalt-phosphate oxygen-evolving compound. Chem Soc Rev. 2009;38(1):109–114. - PubMed
-
- Nocera DG. The artificial leaf. Acc Chem Res. 2012;45(5):767–776. - PubMed
-
- Faunce T, et al. Artificial photosynthesis as a frontier technology for energy sustainability. Energy Envir Sci. 2013;6:1074–1076.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

